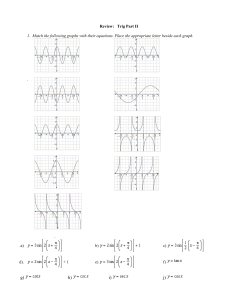
ChemIH Semester II Final Exam Review Multiple Choice Identify the
advertisement

ChemIH Semester II Final Exam Review Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. ____ 1. How many hydrogen atoms are in 5 molecules of isopropyl alcohol, C H O? a. 5 (6.02 10 ) c. 35 b. 5 d. 35 (6.02 10 ) ____ 2. Which of the following elements exists as a diatomic molecule? a. neon c. nitrogen b. lithium d. sulfur ____ 3. How many moles of tungsten atoms are in 4.8 10 atoms of tungsten? a. 8.0 10 moles c. 1.3 10 moles b. 8.0 10 moles d. 1.3 10 moles ____ 4. How many moles of silver atoms are in 1.8 10 a. 3.0 10 c. b. 3.3 10 d. 5. How many molecules are in 2.10 mol CO ? a. 2.53 10 molecules c. b. 3.79 10 molecules d. ____ ____ 6. ____ 7. ____ 8. ____ 9. ____ 10. ____ 11. ____ 12. atoms of silver? 3.0 10 1.1 10 3.49 10 molecules 1.26 10 molecules How many atoms are in 3.5 moles of arsenic atoms? a. 5.8 10 atoms c. 2.1 10 atoms b. 7.5 10 atoms d. 1.7 10 atoms Which of the following is NOT true about atomic mass? a. The atomic mass is 12 g for magnesium. b. The atomic mass is the mass of one mole of atoms. c. The atomic mass is found by checking the periodic table. d. The atomic mass is the number of grams of an element that is numerically equal to the mass in amu. What is true about the molar mass of chlorine gas? a. The molar mass is 35.5 g. b. The molar mass is 71.0 g. c. The molar mass is equal to the mass of one mole of chlorine atoms. d. none of the above The mass of a mole of NaCl is the ____. a. molar mass c. molecular mass b. atomic mass d. gram atomic mass What is the molar mass of (NH ) CO ? a. 144 g c. 96 g b. 138 g d. 78 g What is the number of moles in 432 g Ba(NO ) ? a. 0.237 mol c. 1.65 mol b. 0.605 mol d. 3.66 mol What is the number of moles of beryllium atoms in 36 g of Be? a. 0.25 mol c. 45.0 mol b. 4.0 mol d. 320 mol ____ 13. How many moles of CaBr are in 5.0 grams of CaBr ? a. 2.5 10 mol c. 4.0 10 mol b. 4.2 10 mol d. 1.0 10 mol ____ 14. For which of the following conversions does the value of the conversion factor depend upon the formula of the substance? a. volume of gas (STP) to moles b. density of gas (STP) to molar mass c. mass of any substance to moles d. moles of any substance to number of particles ____ 15. Which combination of temperature and pressure correctly describes standard temperature and pressure, STP? a. 0 C and 101 kPa c. 0 C and 22.4 kPa b. 1 C and 0 kPa d. 100 C and 100 kPa ____ 16. What is the volume, in liters, of 0.500 mol of C H gas at STP? a. 0.0335 L c. 16.8 L b. 11.2 L d. 22.4 L ____ 17. What is the number of moles in 9.63 L of H S gas at STP? a. 0.104 mol c. 3.54 mol b. 0.430 mol d. 14.7 mol ____ 18. Given 1.00 mole of each of the following gases at STP, which gas would have the greatest volume? a. He c. SO b. O d. All would have the same volume. ____ 19. What is conserved in the reaction shown below? H (g) + Cl (g) 2HCl(g) a. mass only c. mass, moles, and molecules only b. mass and moles only d. mass, moles, molecules, and volume ____ 20. In a chemical reaction, the mass of the products ____. a. is less than the mass of the reactants b. is greater than the mass of the reactants c. is equal to the mass of the reactants d. has no relationship to the mass of the reactants ____ 21. In the reaction 2CO(g) + O (g) 2CO (g), what is the ratio of moles of oxygen used to moles of CO produced? a. 1:1 c. 1:2 b. 2:1 d. 2:2 ____ 22. Which of the following is true about the total number of reactants and the total number of products in the reaction shown below? C H (l) + 8O (g) 5CO (g) + 6H O(g) a. 9 moles of reactants chemically change into 11 moles of product. b. 9 grams of reactants chemically change into 11 grams of product. c. 9 liters of reactants chemically change into 11 liters of product. d. 9 atoms of reactants chemically change into 11 atoms of product. ____ 23. How many moles of aluminum are needed to react completely with 1.2 mol of FeO? 2Al(s) + 3FeO(s) 3Fe(s) + Al O (s) a. 1.2 mol c. 1.6 mol b. 0.8 mol d. 2.4 mol ____ 24. When glucose is consumed, it reacts with oxygen in the body to produce carbon dioxide, water, and energy. How many grams of carbon dioxide would be produced if 45 g of C H O completely reacted with oxygen? a. 1.5 g c. 11 g b. 1.8 g d. 66 g ____ 25. Which conversion factor do you use first to calculate the number of grams of CO produced by the reaction of 50.6 g of CH with O ? The equation for the complete combustion of methane is: a. 1 mol CH /16.0 g CH b. 2 mol O /1 mol CO c. 16.0 g CH /1 mol CO d. 44.0 g CO /2 mol CO ____ 26. Which of the following statements is true about the following reaction? 3NaHCO (aq) + C H O (aq) 3CO (g) + 3H O(s) +Na C H O (aq) a. 22.4 L of CO (g) are produced for every liter of C H O (aq) reacted. b. 1 mole of water is produced for every mole of carbon dioxide produced. c. 6.02 10 molecules of Na C H O (aq) are produced for every mole of NaHCO (aq) used. d. 54 g of water are produced for every mole of NaHCO (aq) produced. ____ 27. Which statement is true if 12 mol CO and 12 mol Fe O are allowed to react? 3CO(g) + Fe O (s) 2Fe(s) + 3CO (g) a. The limiting reagent is CO and 8.0 mol Fe will be formed. b. The limiting reagent is CO and 3.0 mol CO will be formed. c. The limiting reagent is Fe O and 24 mol Fe will be formed. d. The limiting reagent is Fe O and 36 mol CO will be formed. ____ 28. Which of the following is NOT true about "yield"? a. The value of the actual yield must be given in order for the percent yield to be calculated. b. The percent yield is the ratio of the actual yield to the theoretical yield. c. The actual yield may be different from the theoretical yield because reactions do not always go to completion. d. The actual yield may be different from the theoretical yield because insufficient limiting reagent was used. ____ 29. According to the kinetic theory, collisions between molecules in a gas ____. a. are perfectly elastic c. never occur b. are inelastic d. cause a loss of total kinetic energy ____ 30. Which of the following statements is part of the kinetic theory? a. The particles of a gas move independently of each other. b. The particles in a gas move rapidly. c. The particles in a gas are relatively far apart. d. all of the above ____ 31. Particles in a gas are best described as ____. a. slow-moving, kinetic, hard spheres b. spheres that are in fixed positions when trapped in a container c. small, hard spheres with insignificant volumes d. hard spheres influenced by repulsive forces from other spheres ____ 32. Which of the following statements is NOT true about the movement of particles in a gas? a. Particles travel in straight-line paths until they collide with other objects. b. Particles usually travel uninterrupted indefinitely. c. Particles fill their containers regardless of the shape or volume of the container. ____ 33. ____ 34. ____ 35. ____ 36. ____ 37. ____ 38. ____ 39. ____ 40. ____ 41. ____ 42. ____ 43. d. The aimless path taken by particles is known as a random walk. What is the SI unit of pressure? a. candela c. pascal b. mole d. newton What is one standard atmosphere of pressure in kilopascals? a. 0 kPa c. 101.3 kPa b. 760 kPa d. 1 kPa Standard conditions when working with gases are defined as ____. a. 0 K and 101.3 kPa c. 0 C and 101.3 kPa b. 0 K and 1 kPa d. 0 C and 1 kPa How does the atmospheric pressure at altitudes below sea level compare with atmospheric pressure at sea level? a. The atmospheric pressure below sea level is higher. b. The atmospheric pressure below sea level is lower. c. The pressures are the same. d. Differences in pressures cannot be determined. What happens to the range of energies of the particles in matter when the temperature is increased? a. The range of energies becomes narrower. b. The range of energies becomes broader. c. The range of energies does not change. d. The range of energies cannot be determined. The average kinetic energy of the particles of a substance ____. a. is not affected by the temperature of the substance b. increases as the temperature of the substance is lowered c. is directly proportional to the temperature of the substance d. is equal to the total energy absorbed by the substance Which states of matter can flow? a. gases only c. gases and liquids only b. liquids only d. gases, liquids, and solids What happens to the temperature of a liquid as it evaporates? a. It increases. c. It does not change. b. It decreases. d. The change cannot be determined. Which are the first particles to evaporate from a liquid? a. particles with the lowest kinetic energy b. particles with the highest kinetic energy c. particles below the surface of the liquid d. All particles evaporate at the same rate. Why does a liquid's rate of evaporation increase when the liquid is heated? a. More molecules have enough energy to overcome the attractive forces holding them in the liquid. b. The average kinetic energy of the liquid decreases. c. The surface area of the liquid is reduced. d. The potential energy of the liquid increases. If a liquid is sealed in a container and kept at constant temperature, how does its vapor pressure change over time? a. It continues to steadily increase. b. It increases at first, then remains constant. c. It increases at first, then decreases. d. It continues to steadily decrease. ____ 44. When the external pressure is 505 kPa, what is the vapor pressure of water at its boiling point? a. 0 kPa c. 505 kPa b. 101.3 kPa d. 1010 kPa ____ 45. Which of the following best describes the motion of the particles in a piece of steel? a. None are moving. c. All are moving. b. A few are moving. d. Most are moving. ____ 46. Which of the following is an example of a phase? a. pressure c. temperature b. water vapor d. triple point ____ 47. How are conditions of pressure and temperature, at which two phases coexist in equilibrium, shown on a phase diagram? a. by a line separating the phases b. by the endpoints of the line segment separating the phases c. by the planar regions between lines in the diagram d. by a triple point on the diagram ____ 48. Why does the pressure inside a container of gas increase if more gas is added to the container? a. There is an increase in the number of collisions between particles and the walls of the container. b. There is an increase in the temperature of the gas. c. There is a decrease in the volume of the gas. d. There is an increase in the force of the collisions between the particles and the walls of the container. ____ 49. If the volume of a container of gas is reduced, what will happen to the pressure inside the container? a. The pressure will increase. b. The pressure will not change. c. The pressure will decrease. d. The pressure depends on the type of gas. ____ 50. As the temperature of the gas in a balloon decreases, which of the following occurs? a. The volume of the balloon increases. b. The average kinetic energy of the gas decreases. c. The gas pressure inside the balloon increases. d. all of the above ____ 51. If 4 moles of gas are added to a container that already holds 1 mole of gas, how will the pressure change inside the container? a. The pressure will be five times higher. b. The pressure will double. c. The pressure will be four times higher. d. The pressure will not change. ____ 52. Which of these changes would NOT cause an increase in the pressure of a contained gas? a. The volume of the container is increased. b. More of the gas is added to the container. c. The temperature is increased. d. The average kinetic energy of the gas in increased. ____ 53. If a balloon is heated, what happens to the volume of the air in the balloon if the pressure is constant? a. It increases. c. It decreases. b. It stays the same. d. The change cannot be predicted. ____ 54. If a sealed syringe is plunged into cold water, in which direction will the syringe piston slide? a. in c. No movement will occur. b. out d. The direction cannot be predicted. ____ 55. In general, for a gas at a constant volume, ____. a. the pressure of the gas is inversely proportional to its temperature in kelvins b. the volume of the gas is inversely proportional to its temperature in kelvins c. the volume of the gas is directly proportional to its temperature in kelvins d. the pressure of the gas is directly proportional to its temperature in kelvins ____ 56. The combined gas law relates which of the following? a. pressure and volume only c. volume and temperature only b. temperature and pressure only d. temperature, pressure, and volume ____ 57. Which law can be used to calculate the number of moles of a contained gas? a. Boyle’s law c. ideal gas law b. combined gas law d. Charles’s law ____ 58. Which of the following is constant for 1 mole of any ideal gas? a. PVT c. b. d. ____ 59. What happens to the partial pressure of oxygen in a sample of air if the temperature is increased? a. It increases. c. It decreases. b. It stays the same. d. The change cannot be determined. ____ 60. What happens to the energy produced by burning gasoline in a car engine? a. The energy is lost as heat in the exhaust. b. The energy is transformed into work to move the car. c. The energy heats the parts of the engine. d. all of the above ____ 61. A piece of metal is heated, then submerged in cool water. Which statement below describes what happens? a. The temperature of the metal will increase. b. The temperature of the water will increase. c. The temperature of the water will decrease. d. The temperature of the water will increase and the temperature of the metal will decrease. ____ 62. How does a calorie compare to a joule? a. A calorie is smaller than a joule. c. A calorie is equal to a joule. b. A calorie is larger than a joule. d. The relationship cannot be determined. ____ 63. Which of the following is NOT a form of energy? a. light c. heat b. pressure d. electricity ____ 64. If heat is released by a chemical system, an equal amount of heat will be ____. a. absorbed by the surroundings c. released by the surroundings b. absorbed by the universe d. released by the universe ____ 65. Which of the following is transferred due to a temperature difference? a. chemical energy c. electrical energy b. mechanical energy d. heat ____ 66. In an exothermic reaction, the energy stored in the chemical bonds of the reactants is ____. a. equal to the energy stored in the bonds of the products b. greater than the energy stored in the bonds of the products c. less than the energy stored in the bonds of the products d. less than the heat released ____ 67. When your body breaks down sugar completely, how much heat is released compared to burning the same amount of sugar in a flame? a. The body releases more heat. b. The body releases less heat. c. The body releases the same amount of heat. d. The body releases no heat. ____ 68. How many joules are in 148 calories? (1 cal = 4.18 J) a. 6.61 J c. 148 J b. 35.4 J d. 619 J ____ 69. What is the amount of heat required to raise the temperature of 200.0 g of aluminum by 10 C? (specific heat of aluminum = 0.21 ____ 70. ____ 71. ____ 72. ____ 73. ____ 74. ____ 75. ____ 76. ) a. 420 cal c. 42,000 cal b. 4200 cal d. 420,000 cal By what quantity must the heat capacity of an object be divided to obtain the specific heat of that material? a. its mass c. its temperature b. its volume d. its energy What does the symbol H stand for? a. the specific heat of a substance b. the heat capacity of a substance c. the heat of reaction for a chemical reaction d. one Calorie given off by a reaction On what principle does calorimetry depend? a. Hess's law c. law of enthalpy b. law of conservation of energy d. law of multiple proportions A chunk of ice whose temperature is –20 C is added to an insulated cup filled with water at 0 C. What happens in the cup? a. The ice melts until it reaches the temperature of the water. b. The water cools until it reaches the temperature of the ice. c. Some of the water freezes, so the chunk of ice gets larger. d. none of the above To calculate the amount of heat absorbed as a substance melts, which of the following information is NOT needed? a. the mass of the substance c. the change in temperature b. the specific heat of the substance d. the density of the sample When an acid reacts with a base, what compounds are formed? a. a salt only c. metal oxides only b. water only d. a salt and water What is the formula for phosphoric acid? a. H PO c. HPO b. H PO d. HPO ____ 77. What is an acid according to Arrhenius? a. a substance that ionizes to yield protons in aqueous solution b. a substance that is a hydrogen ion donor c. a substance that accepts an electron pair d. a substance that is a hydrogen ion acceptor ____ 78. Which of these is an Arrhenius base? a. LiOH b. NH c. H PO d. CH COOH ____ 79. What is transferred between a conjugate acid-base pair? a. an electron c. a hydroxide ion b. a proton d. a hydronium ion ____ 80. In the reaction of aluminum bromide with ionized sodium bromide, which compound is the Lewis acid? a. aluminum bromide c. sodium ion b. bromide ion d. None are Lewis acids. ____ 81. What type of acid is sulfuric acid? a. monoprotic c. triprotic b. diprotic d. none of the above ____ 82. Which compound can act as both a Brønsted-Lowry acid and a Brønsted-Lowry base? a. water c. sodium hydroxide b. ammonia d. hydrochloric acid ____ 83. In the reaction CO +H O HCO + OH , the carbonate ion is acting as a(n) ____. a. Arrhenius base c. Brønsted-Lowry base b. Arrhenius acid d. Brønsted-Lowry acid ____ 84. What is the charge on the hydronium ion? a. 2– c. 0 b. 2– d. 1+ ____ 85. What is pH? a. the negative logarithm of the hydrogen ion concentration b. the positive logarithm of the hydrogen ion concentration c. the negative logarithm of the hydroxide ion concentration d. the positive logarithm of the hydroxide ion concentration ____ 86. Which type of solution is one with a pH of 8? a. acidic b. basic c. neutral d. The type varies, depending on the solution. ____ 87. What characterizes a strong acid or base? a. polar covalent bonding b. complete ionization in water c. ionic bonding d. presence of a hydroxide or hydrogen ion ____ 88. If an acid has a [H+] = 1.6 10 , what is the acidity of the solution? a. acidic c. neutral b. basic d. The answer cannot be determined. ____ 89. Which acid has the greatest acid dissociation constant? a. nitric acid c. carbonic acid b. acetic acid d. boric acid ____ 90. The process of adding a known amount of solution of known concentration to determine the concentration of another solution is called ____. a. neutralization c. titration b. hydrolysis d. buffer capacity ____ 91. In a titration, when the number of moles of hydrogen ions equals the number of moles of hydroxide ions, what is said to have happened? ____ 92. ____ 93. ____ 94. ____ 95. ____ 96. ____ 97. ____ 98. ____ 99. a. The equivalence point has been reached. b. The end point has been reached. c. The point of neutralization has been reached. d. all the above. Which is the most susceptible to damage from ionizing radiation? a. soft tissue c. wood b. paper d. lead A beta particle is a(n) ____. a. photon c. helium nucleus b. electron d. hydrogen nucleus What is the change in atomic mass when an atom emits a beta particle? a. decreases by 2 c. remains the same b. decreases by 1 d. increases by 1 What is the change in atomic mass when an atom emits gamma radiation? a. decreases by 2 c. remains the same b. decreases by 1 d. increases by 1 What is the change in the atomic number when an atom emits an alpha particle? a. decreases by 2 c. increases by 1 b. decreases by 1 d. increases by 2 What is the change in atomic number when an atom emits a beta particle? a. decreases by 2 c. increases by 2 b. decreases by 1 d. increases by 1 What is the change in atomic number caused by the emission of gamma radiation? a. decreases by 2 c. remains the same b. decreases by 1 d. increases by 1 Which symbol is used for an alpha particle? a. He c. He b. He d. He ____ 100. Which of the following materials is necessary to stop an alpha particle? a. three feet of concrete c. single sheet of aluminum foil b. three inches of lead d. single sheet of paper ____ 101. Which of the following materials is most effective for stopping gamma radiation? a. several cm of lead c. single sheet of aluminum foil b. one cm of water d. single sheet of paper ____ 102. What does the band of stability for atomic nuclei refer to? a. the ratio of protons to neutrons b. the ratio of neutrons to protons c. the ratio of beta particles to neutrinos d. the ratio of alpha particles to beta particles ____ 103. If an isotope decays by the process of beta emission, ____. a. the mass number changes b. the atomic number changes c. protons are given off d. the number of neutrons remains the same ____ 104. What particle is needed to complete this nuclear reaction? Rn Po + _____ a. He c. H b. e d. n ____ 105. What is the approximate half-life of carbon-14? a. hundreds of years c. millions of years b. thousands of years d. billions of years ____ 106. Which of the following naturally occurring radioisotopes would be most useful in dating objects thought to be millions of years old? a. carbon-14; t = 5.73 10 years b. potassium-40; t = 1.28 10 years c. thorium-234; t = 25 days d. radon-222; t = 3.8 days ____ 107. The production of carbon-14 ____. a. takes place in the upper atmosphere b. is mostly due to fallout from nuclear explosions c. occurs to a large extent in nuclear reactors d. is caused by photosynthesis in plants ____ 108. Controlled nuclear chain reactions ____. a. take place in nuclear reactors b. are always fusion reactions c. never produce radioactive by-products d. are characteristic of atomic bombs ____ 109. Which type of coolant(s) usually is (are) used to remove heat from a nuclear reactor core? a. water only c. liquid sodium or water b. liquid sodium only d. CFCs ____ 110. What does neutron absorption accomplish in a nuclear reactor? a. It slows down the reaction. b. It speeds up the reaction. c. It increases the rate of heat absorption. d. It recycles the fuel. ____ 111. Control rods made of ____. a. carbon c. plutonium b. liquid sodium d. cadmium ____ 112. What substances are used as moderators in a nuclear reactor? a. carbon and water c. plutonium and neptunium b. liquid sodium and water d. cadmium or other metal ____ 113. Which of the following statements is correct? a. Water is used to moderate (slow down) neutrons in a nuclear reactor. b. Carbon control rods are used to absorb neutrons in a nuclear fission reaction. c. A very high temperature is required to initiate a nuclear fission reaction. d. The energy released from the sun is the result of a nuclear fission reaction. ____ 114. What instrument is used routinely to check a person's exposure to radiation? a. Geiger counter c. film badge b. scintillation counter d. moderating rod ____ 115. What is the main detector of a Geiger counter? a. ionizable gas in a metal tube c. plates of ionizable plastic b. phosphor-covered surface d. potassium metal surface ____ 116. Radiation therapy is used to ____. a. study reaction mechanisms b. detect elements c. treat cancer d. initiate neutron activation analysis Short Answer 117. How many representative particles are in 1.45 g of a molecular compound with a molar mass of 237 g? 118. Find the mass in grams of 3.10 119. Find the mass, in grams, of 1.40 10 molecules of F . 10 molecules of N . 120. What is the percent by mass of hydrogen in aspirin, C H O ? 121. If a total of 13.5 mol of NaHCO and 4.5 mol of C H O react, how many moles of CO and Na C H O will be produced? 3NaHCO (aq) + C H O (aq) 3CO (g) + 3H O(s) +Na C H O (aq) 122. If 8.6 L of H reacted with 4.3 L of O at STP, what is the volume of the gaseous water collected (assuming that none of it condenses)? 2H (g) + O (g) 2H O(g) 123. Assuming STP and a stoichiometric amount of NH and NO in an expandable container originally at 15 L, what is the final volume if the reaction goes to completion? 4NH (g) + 6NO(g) 5N (g) + 6H O(g) 124. How many grams of CO are needed to react with an excess of Fe O to produce 209.7 g Fe? Fe O (s) + 3CO(g) 3CO (g) + 2Fe(s) 125. How many liters of O are needed to react completely with 45.0 L of H S at STP? 2H S(g) + 3O (g) 2SO (g) + 2H O(g) 126. If 5.0 g of H are reacted with excess CO, how many grams of CH OH are produced, based on a yield of 86%? CO(g) + 2H (g) CH OH(l) 127. For the reaction 2Na(s) + Cl (g) Na and 13.0 L of Cl (at STP)? 2NaCl(s), how many grams of NaCl could be produced from 103.0 g of 128. Solid sodium reacts violently with water, producing heat, hydrogen gas, and sodium hydroxide. How many molecules of hydrogen gas are formed when 48.7 g of sodium are added to water? 2Na + 2H O 2NaOH + H 129. The decomposition of potassium chlorate yields oxygen gas. If the yield is 95%, how many grams of KClO are needed to produce 10.0 L of O ? 2KClO (s) 2KCl(s) + 3O (g) 130. What is a pressure of 0.520 atm equal to in mm of Hg? 131. The vapor pressure of 10 mL of ethanol at 20 C is 5.85 kPa. What is the vapor pressure of 20 mL of ethanol at the same temperature? 132. What is the pressure (in atm) at the normal boiling point of water? 133. What is the angle measurement in cubic, tetragonal, and orthorhombic crystal systems? 134. A balloon filled with helium has a volume of 30.0 L at a pressure of 100 kPa and a temperature of 15.0 C. What will the volume of the balloon be if the temperature is increased to 80.0 C and the pressure remains constant? 135. A gas has a pressure of 710 kPa at 227 C. What will its pressure be at 27 C, if the volume does not change? 136. A gas storage tank has a volume of 3.5 10 m when the temperature is 27 C and the pressure is 101 kPa. What is the new volume of the tank if the temperature drops to –10 C and the pressure drops to 95 kPa? 137. How many moles of N are in a flask with a volume of 250 mL at a pressure of 300.0 kPa and a temperature of 300.0 K? 138. What is the pressure exerted by 32 g of O in a 22.0-L container at 30.0 C? 139. Use Graham’s law to calculate how much faster fluorine gas, F , will effuse than chlorine gas, Cl , will. The molar mass of F = 38.0; the molar mass of Cl = 70.9. 140. How many joules are there in 215 calories? (1 cal = 4.184 J) 141. How much heat is required to raise the temperature of 5.5 aluminum = 0.21 10 g of aluminum by 10 C? (specific heat of ) 142. The specific heat capacity of graphite is 0.71 . Calculate the energy required to raise the temperature of 750 g of graphite by 160 C. 143. It takes 770 joules of energy to raise the temperature of 50.0 g of mercury by 110 C. What is the specific heat of mercury? 144. When 64.0 g of methanol (CH OH) is burned, 1454 kJ of energy is produced. What is the heat of combustion for methanol? 145. How much heat is required to melt 1.6 moles of NaCl ( H = 30.2 kJ/mol) at its melting point? 146. A certain substance with a molar mass of 43 g has a heat of fusion of 48 cal/g. How many calories are needed to melt 7.2 kg of the substance? 147. Suppose a substance has a heat of fusion equal to 45 cal/g and a specific heat of 0.75 in the liquid state. If 5.0 kcal of heat are applied to a 50-g sample of the substance at a temperature of 24 C, what will its new temperature be? What state will the sample be in? (melting point of the substance = 27 C; specific heat of the solid = 0.48 ; boiling point of the substance = 700 C) 148. A substance releases 496 kJ of heat as 2.60 moles condense from a gas into a liquid. What is the molar heat of vaporization of the substance? 149. Consider a 67-g chunk of ice ( H = 6.0 kJ/mol) in a beaker immersed in a water bath. To produce just enough heat to melt the ice, how many moles of solid NaOH ( H = –445.1 kJ/mol) must you dissolve in the water bath? 150. Use the information below to calculate H for the following reaction. 2NO (g) N O (g) 2N (g) + 2O (g) 2NO (g) H = 67.7 kJ N (g) + 2O (g) N O (g) H = 9.7 kJ 151. What is the ion-product constant for water? 152. Calculate the hydrogen-ion concentration [H ] for an aqueous solution in which [OH ] is 1 this solution acidic, basic, or neutral? 10 mol/L. Is 153. If the pH is 9, what is the concentration of hydroxide ion? 154. A liter of impure water has 10 sample of water? mol of hydroxide ions. What is the concentration of hydronium ions in this 155. If the hydroxide-ion concentration is 1 10 M, what is the pH of the solution? 156. What is the hydrogen-ion concentration if the pH is 3.7? 157. What is the hydrogen-ion concentration if the acid dissociation constant is 0.000 001 and the acid concentration is 0.01M? 158. A 0.500M solution of a weak acid, HX, is only partially ionized. The [H ] was found to be 4.02 10 M. Find the dissociation constant for this acid. 159. What is the pH if the hydrogen-ion concentration is 6.8 10 M? 160. What is the acid dissociation constant of a weak acid if a concentration of 0.3M gives a hydrogen-ion concentration of 0.001M? 161. Calculate the acid dissociation constant of a weak monoprotic acid if a 0.5M solution of this acid gives a hydrogen-ion concentration of 0.000 1M? 162. Complete the following nuclear reaction: U He + Th 163. If the half-life of a radioactive material is 8 years, how many years will it take for one half of the original amount of material to decay? 164. If the half-life of sodium-24 is 15 hours, how much remains from a 10.0-g sample after 60 hours? 165. What is the half-life of iodine-131 if, after 24 days, 0.125 g remains from a 1.00-g starting sample? 166. The concentration of carbon-14 in a piece of wood from an ancient burial mound indicates that two half-lives of this radioisotope have passed. If the half-life (t ) for carbon-14 is 5730 years, approximately how many years ago did this sample of wood die? ChemIH Semester II Final Exam Review Answer Section MULTIPLE CHOICE 1. ANS: OBJ: 2. ANS: 3. ANS: OBJ: 4. ANS: OBJ: 5. ANS: OBJ: 6. ANS: OBJ: 7. ANS: 8. ANS: 9. ANS: 10. ANS: OBJ: 11. ANS: 12. ANS: 13. ANS: 14. ANS: 15. ANS: 16. ANS: 17. ANS: 18. ANS: 19. ANS: STO: 20. ANS: STO: 21. ANS: 22. ANS: 23. ANS: OBJ: 24. ANS: OBJ: 25. ANS: OBJ: 26. ANS: OBJ: 27. ANS: 28. ANS: 29. ANS: STO: C 10.1.2 C B 10.1.2 A 10.1.2 D 10.1.2 C 10.1.2 A B A C 10.1.4 C B A C A B B D D SC.B.1.4.2 C SC.B.1.4.2 C A B 12.2.1 D 12.2.2 A 12.2.2 B 12.2.2 A D A SC.B.1.4 DIF: L1 REF: p. 291, p. 292 DIF: L1 DIF: L2 REF: p. 290 OBJ: 10.1.2 REF: p. 290, p. 291 DIF: L2 REF: p. 290, p. 291 DIF: L2 REF: p. 291, p. 292 DIF: L2 REF: p. 291, p. 292 DIF: DIF: DIF: DIF: L2 L2 L1 L2 REF: REF: REF: REF: p. 294 OBJ: 10.1.3 p. 294 OBJ: 10.1.3 p. 295 OBJ: 10.1.4 p. 295, p. 296 DIF: DIF: DIF: DIF: DIF: DIF: DIF: DIF: DIF: L2 L2 L2 L2 L1 L2 L2 L3 L1 REF: REF: REF: REF: REF: REF: REF: REF: REF: p. 299 p. 299 p. 299 p. 297 p. 300 p. 301 p. 301 p. 301 p. 356 OBJ: OBJ: OBJ: OBJ: OBJ: OBJ: OBJ: OBJ: OBJ: 10.2.1 10.2.1 10.2.1 10.2.1 10.2.2 10.2.2 10.2.2 10.2.2 12.1.2 DIF: L1 REF: p. 356 OBJ: 12.1.2 DIF: L1 DIF: L2 DIF: L1 REF: p. 356 OBJ: 12.1.2 REF: p. 356 OBJ: 12.1.2 REF: p. 359, p. 360 DIF: L2 REF: p. 360, p. 361, p. 362 DIF: L2 REF: p. 360, p. 361, p. 362 DIF: L2 REF: p. 363, p. 364, p. 365, p. 366 DIF: L2 DIF: L1 DIF: L1 REF: p. 369 REF: p. 372 REF: p. 385 OBJ: 12.3.1 OBJ: 12.3.2 OBJ: 13.1.1 30. ANS: STO: 31. ANS: STO: 32. ANS: STO: 33. ANS: 34. ANS: 35. ANS: 36. ANS: STO: 37. ANS: STO: 38. ANS: STO: 39. ANS: STO: 40. ANS: STO: 41. ANS: STO: 42. ANS: STO: 43. ANS: 44. ANS: 45. ANS: STO: 46. ANS: STO: 47. ANS: STO: 48. ANS: STO: 49. ANS: 50. ANS: 51. ANS: 52. ANS: OBJ: 53. ANS: 54. ANS: 55. ANS: 56. ANS: 57. ANS: 58. ANS: 59. ANS: OBJ: 60. ANS: STO: D SC.B.2.4.1 C SC.A.1.4.3 B SC.A.1.4.3 C C C A SC.D.1.4 B SC.B.1.4.3 C SC.B.1.4.3 C SC.A.1.4.3 B SC.A.1.4.3 B SC.A.1.4.3 A SC.A.1.4.3 B C C SC.A.1.4.2 B SC.A.1.4.3 A SC.A.1.4.3 A SC.A.1.4.2 A B A A 14.1.2 A A D D C B A 14.2.1, 14.4.1 D SC.B.1.4.2 DIF: L1 REF: p. 385 OBJ: 13.1.1 DIF: L2 REF: p. 385 OBJ: 13.1.1 DIF: L3 REF: p. 385 OBJ: 13.1.1 DIF: DIF: DIF: DIF: REF: REF: REF: REF: OBJ: OBJ: OBJ: OBJ: L1 L1 L1 L2 p. 387 p. 387 p. 387 p. 386 13.1.2 13.1.2 13.1.2 13.1.2 DIF: L2 REF: p. 388 OBJ: 13.1.3 DIF: L2 REF: p. 389 OBJ: 13.1.3 DIF: L2 REF: p. 390 OBJ: 13.2.1 DIF: L1 REF: p. 391 OBJ: 13.2.2 DIF: L1 REF: p. 391 OBJ: 13.2.2 DIF: L2 REF: p. 391 OBJ: 13.2.2 DIF: L2 DIF: L2 DIF: L2 REF: p. 392 REF: p. 394 REF: p. 396 OBJ: 13.2.3 OBJ: 13.2.4 OBJ: 13.3.1 DIF: L1 REF: p. 402 OBJ: 13.4.2 DIF: L2 REF: p. 402 OBJ: 13.4.2 DIF: L1 REF: p. 415 OBJ: 14.1.2 DIF: DIF: DIF: DIF: L1 L1 L2 L2 REF: REF: REF: REF: p. 416 OBJ: 14.1.2 p. 417 OBJ: 14.1.2 p. 415 OBJ: 14.1.2 p. 415, p. 416, p. 417 DIF: DIF: DIF: DIF: DIF: DIF: DIF: L1 L2 L2 L1 L1 L2 L2 REF: REF: REF: REF: REF: REF: REF: p. 420 p. 420 p. 422 p. 424 p. 426 p. 429 p. 422, p. 432 DIF: L1 REF: p. 505 OBJ: OBJ: OBJ: OBJ: OBJ: OBJ: 14.2.1 14.2.1 14.2.1 14.2.2 14.3.1 14.3.1 OBJ: 17.1.1 61. ANS: STO: 62. ANS: 63. ANS: STO: 64. ANS: STO: 65. ANS: STO: 66. ANS: STO: 67. ANS: 68. ANS: 69. ANS: STO: 70. ANS: OBJ: 71. ANS: 72. ANS: 73. ANS: STO: 74. ANS: STO: 75. ANS: 76. ANS: 77. ANS: 78. ANS: 79. ANS: 80. ANS: 81. ANS: 82. ANS: 83. ANS: 84. ANS: 85. ANS: 86. ANS: 87. ANS: 88. ANS: 89. ANS: 90. ANS: OBJ: 91. ANS: OBJ: 92. ANS: OBJ: 93. ANS: STO: 94. ANS: STO: D SC.B.1.4.6 B B SC.A.1.4.3 A SC.B.1.4.2 D SC.B.1.4.6 B SC.A.1.4.4 C D A SC.B.1.4.6 A 17.1.3 C B C SC.B.1.4.6 D SC.A.1.4.3 D B A A B A B A C D A B B B A C 19.4.1 D 19.4.2 A 25.1.2 B SC.A.2.4.3 C SC.A.2.4.3 DIF: L1 REF: p. 506 OBJ: 17.1.1 DIF: L1 DIF: L1 REF: p. 506 REF: p. 505 OBJ: 17.1.1 OBJ: 17.1.1 DIF: L1 REF: p. 506 OBJ: 17.1.1 DIF: L1 REF: p. 506 OBJ: 17.1.1 DIF: L2 REF: p. 506 OBJ: 17.1.1 DIF: L1 DIF: L1 DIF: L1 REF: p. 507 REF: p. 507 REF: p. 508 OBJ: 17.1.2 OBJ: 17.1.3 OBJ: 17.1.3 DIF: STO: DIF: DIF: DIF: REF: p. 509, p. 510 L2 SC.A.1.4.4 L1 L2 L2 REF: p. 514 REF: p. 511 REF: p. 512 OBJ: 17.2.1 OBJ: 17.2.1 OBJ: 17.2.1 DIF: L2 REF: p. 521 OBJ: 17.3.1 DIF: DIF: DIF: DIF: DIF: DIF: DIF: DIF: DIF: DIF: DIF: DIF: DIF: DIF: DIF: DIF: REF: REF: REF: REF: REF: REF: REF: REF: REF: REF: REF: REF: REF: REF: REF: REF: L1 L1 L1 L1 L1 L2 L2 L2 L2 L1 L1 L1 L1 L2 L3 L1 DIF: L1 p. 587 OBJ: p. 588 OBJ: p. 588 OBJ: p. 589 OBJ: p. 591 OBJ: p. 592 OBJ: p. 588 OBJ: p. 591 OBJ: p. 590 OBJ: p. 594 OBJ: p. 596 OBJ: p. 597 OBJ: p. 605 OBJ: p. 610 OBJ: p. 607 OBJ: p. 613, p. 614, p. 615 19.1.1 19.1.1 19.1.2 19.1.2 19.1.2 19.1.2 19.1.2 19.1.2 19.1.2 19.2.1 19.2.2 19.2.2 19.3.1 19.3.3 19.3.3 REF: p. 613, p. 614, p. 615 DIF: L1 REF: p. 800, p. 802 STO: SC.A.2.4.1, SC.A.2.4.3 DIF: L1 REF: p. 801 OBJ: 25.1.2 DIF: L1 REF: p. 801 OBJ: 25.1.2 95. ANS: STO: 96. ANS: STO: 97. ANS: STO: 98. ANS: STO: 99. ANS: STO: 100. ANS: STO: 101. ANS: STO: 102. ANS: OBJ: 103. ANS: STO: 104. ANS: STO: 105. ANS: STO: 106. ANS: OBJ: 107. ANS: STO: 108. ANS: STO: 109. ANS: STO: 110. ANS: STO: 111. ANS: STO: 112. ANS: STO: 113. ANS: OBJ: 114. ANS: STO: 115. ANS: STO: 116. ANS: STO: C DIF: L1 SC.A.2.4.3 A DIF: L2 SC.A.2.4.1, SC.A.2.4.3 D DIF: L2 SC.A.2.4.1, SC.A.2.4.3 C DIF: L2 SC.A.2.4.1 D DIF: L2 SC.A.2.4.3 D DIF: L2 SC.A.2.4.3 A DIF: L2 SC.A.2.4.3 B DIF: L2 25.2.1 STO: SC.A.2.4.3 B DIF: L2 SC.A.2.4.1, SC.A.2.4.3 A DIF: L2 SC.A.2.4.3 B DIF: L1 SC.A.2.4.3 B DIF: L2 25.2.2 STO: SC.A.2.4.3 A DIF: L3 SC.A.2.4.1 A DIF: L2 SC.A.2.4.4 C DIF: L1 SC.A.2.4.4 A DIF: L2 SC.A.2.4.4 D DIF: L2 SC.A.2.4.4 A DIF: L2 SC.A.2.4.4 A DIF: L2 25.3.3 STO: SC.A.2.4.4 C DIF: L1 SC.A.2.4.4 A DIF: L2 SC.A.2.4.4 C DIF: L1 SC.B.1.4.1 SHORT ANSWER 117. ANS: REF: p. 802 OBJ: 25.1.2 REF: p. 800 OBJ: 25.1.1, 25.1.2 REF: p. 801 OBJ: 25.1.2 REF: p. 802 OBJ: 25.1.2 REF: p. 800 OBJ: 25.1.2 REF: p. 800 OBJ: 25.1.2 REF: p. 802 OBJ: 25.1.2 REF: p. 803, p. 804 REF: p. 801 OBJ: 25.2.1 REF: p. 801 OBJ: 25.2.1 REF: p. 805 OBJ: 25.2.2 REF: p. 804, p. 805 REF: p. 806 OBJ: 25.2.3 REF: p. 810 OBJ: 25.3.1 REF: p. 811 OBJ: 25.3.3 REF: p. 811 OBJ: 25.3.3 REF: p. 811 OBJ: 25.3.3 REF: p. 811 OBJ: 25.3.3 REF: p. 810, p. 811 REF: p. 817 OBJ: 25.4.1 REF: p. 817 OBJ: 25.4.1 REF: p. 819 OBJ: 25.4.2 1.45 g 1.00 mol/237 g 6.02 = 3.68 10 molecules 10 molecules/1.00 mol DIF: L2 REF: p. 292 118. ANS: 3.10 10 molecules 1 mol F /6.02 = 19.6 g F OBJ: 10.1.2 10 molecules 38.0 g F /1 mol F DIF: L2 REF: p. 297 OBJ: 10.2.1 119. ANS: 1.40 10 molecules N (1.00 mol N /6.02 10 molecules N ) = 6.51 g DIF: L3 REF: p. 291, p. 297 120. ANS: 8.00 g H /180 g C H O 100% = 4.44% H DIF: L3 REF: p. 307 121. ANS: 13.5 mol of CO ; 4.5 mol of Na C H O (28.0 g N /1 mol N ) OBJ: 10.1.2, 10.2.1 OBJ: 10.3.1 DIF: L1 REF: p. 359 OBJ: 12.2.1 122. ANS: 8.6 L H / (22.4 L/1 mol) 2 mol H O/2 mol H 22.4 L/ 1 mol = 8.6 L H O DIF: L2 REF: p. 363 OBJ: 12.2.2 123. ANS: 15 L of given reactants 11 L of products/10 L of reactants = 16.5 L DIF: L3 124. ANS: 209.7 g Fe DIF: L2 125. ANS: 45.0 L H S REF: p. 363 1 mol Fe/55.85 g Fe OBJ: 12.2.2 3 mol CO/2 mol Fe REF: p. 371 1 mol H S/22.4 L H S 28.01 g CO/1 mol CO = 157.8 g CO OBJ: 12.3.1 3 mol O /2 mol H S 22.4 L O /1 mol O = 67.5 L O DIF: L3 REF: p. 371 OBJ: 12.3.1 126. ANS: Theoretical yield: 5.0 g H 1 mol H /2.0 g H 1 mol CH OH/2 mol H 32 g CH OH/1 mol CH OH = 40 g CH OH 40 g CH OH 86% = 34 g CH OH DIF: L3 127. ANS: 13.0 L Cl REF: p. 371 OBJ: 12.3.1 1 mol Cl /22.4 L Cl = 0.580 mol Cl 103.0 g Na 1 mol Na/23 g Na = 4.48 mol Na Cl is limiting reagent: 0.580 mol Cl 2 mol NaCl/1 mol Cl = 1.16 mol NaCl 1.16 mol NaCl 58 g NaCl/1 mol NaCl = 67.3 g NaCl DIF: L3 REF: p. 371 OBJ: 12.3.1 128. ANS: Assume the sodium is limiting: 48.7 g Na 1 mol Na/23.0 g Na 1 mol H /2 mol Na (6.02 = 6.37 10 REF: p. 374 760 mm Hg / 1 atm = 395 mm Hg REF: p. 387 OBJ: 13.1.2 DIF: L3 132. ANS: 101.3 kPa REF: p. 392 OBJ: 13.2.3 1 atm/101.3 kPa = 1.00 atm DIF: L2 133. ANS: 90 REF: p. 387, p. 395 DIF: L2 134. ANS: REF: p. 397 V =V = 30.0 L 27 C + 273 = 300 K ; = =P OBJ: 13.2.4 OBJ: 13.3.2 = 34.3 L DIF: L2 REF: p. 421 135. ANS: 227 C + 273 = 500 K 710 kPa 122.6 g KClO /1mol KClO OBJ: 12.3.2 DIF: L2 131. ANS: 5.85 kPa = molecules H )/1 mol H molecules H DIF: L3 REF: p. 371 OBJ: 12.3.1 129. ANS: 10.0 L 100%/95% = 10.5 L theoretical yield 10.5 L O 1 mol O /22.4 L O 2 mol KClO /3 mol O = 38.4 g KClO DIF: L3 130. ANS: 0.520 atm 10 OBJ: 14.2.1 P = 470 kPa DIF: L3 REF: p. 421 136. ANS: T = 27 C + 273 = 300 K; P = 101 kPa T = –10 C + 273 = 263 K; P = 95 kPa V =P V V = 3.26 = (101 kP) 10 m ) 10 m DIF: L3 137. ANS: REF: p. 424 250 mL OBJ: 14.2.2 = 0.25 L n=P = DIF: L2 138. ANS: = 0.030 mol REF: p. 427 32 g O P= (3.5 OBJ: 14.2.1 OBJ: 14.3.1 = 1 mol O = = 110 kPa DIF: L2 139. ANS: REF: p. 427 OBJ: 14.3.1 = 1.4 DIF: L2 140. ANS: 215 cal REF: p. 436 4.184 = 9.00 OBJ: 14.4.2 10 J DIF: L2 REF: p. 507 OBJ: 17.1.2 141. ANS: Heat energy = mass specific heat temperature change = 550 g = 1.2 DIF: L2 142. ANS: 0.21 10 C cal REF: p. 508 OBJ: 17.1.3 STO: SC.B.1.4.6 H = 750 g 0.71 DIF: L2 143. ANS: 160 C = 85,000 J REF: p. 512 Specific heat = DIF: L2 144. ANS: OBJ: 17.2.1 STO: SC.B.1.4.3 OBJ: 17.2.1 STO: SC.B.1.4.3 = 0.14 REF: p. 512 H= = 727 kJ/mol DIF: L2 REF: p. 517 145. ANS: 1.6 mol 30.2 kJ/mol = 48 kJ OBJ: 17.2.2 STO: SC.B.1.4.3 DIF: L2 146. ANS: 48 cal/g 7.2 kg OBJ: 17.3.1 STO: SC.A.1.4.3 OBJ: 17.3.1 STO: SC.A.1.4.3 DIF: L2 147. ANS: 50 g 0.48 REF: p. 521 1000 g/kg = 350,000 cal REF: p. 521 3.0 C = 72 cal to raise the temperature of the solid to 27 C 50 g 45 cal/g = 2250 cal to melt the sample 2250 cal + 72 cal = 2322 cal 5000 cal – 2322 cal = 2678 cal remaining T= = 71 C 71 C + 27 C = 98 C The substance is in a liquid state. DIF: L3 148. ANS: H = REF: p. 521 OBJ: 17.3.1 STO: SC.A.1.4.3 = 191 kJ/mol DIF: L2 REF: p. 524 OBJ: 17.3.2 STO: SC.A.1.4.3 149. ANS: Heat to melt ice comes from heat released by the dissolving of NaOH. Amount of NaOH = 67 g H O = 0.050 mol NaOH DIF: L3 REF: p. 520, p. 521 STO: SC.A.1.4.3 150. ANS: 2NO (g) N (g) + 2O (g) H = –67.7 kJ N (g) + 2O (g) N O (g) H = 9.7 kJ ___________________________________________ 2NO (g) N O (g) H = –58 kJ DIF: L3 151. ANS: 10 M REF: p. 528 OBJ: 17.4.2 DIF: L1 152. ANS: K = [H ] REF: p. 595 OBJ: 19.2.1 DIF: L3 153. ANS: 10 M REF: p. 595 OBJ: 19.2.1 DIF: L1 154. ANS: 10 M REF: p. 598 OBJ: 19.2.2 DIF: L1 155. ANS: K = [H ] REF: p. 598 OBJ: 19.2.2 [OH ] [H ] = = = 1 10 mol/L The solution is acidic. [OH ] [H ] = = = 1 10- 2 mol/L pH = - log [H ] = - log [ 1 10- 2 mol/L] = 2.0 DIF: L2 REF: p. 598 156. ANS: –log [H ] = pH = 3.7 OBJ: 19.2.2 OBJ: 17.3.2 log [H ] = –3.7 [H ] = antilog(–3.7) [H ] = 0.000 20M DIF: L2 157. ANS: 10 M REF: p. 600 OBJ: 19.2.2 DIF: L2 REF: p. 607 OBJ: 19.3.2 158. ANS: [HX] = 0.500M – 4.02 10 M = 0.496M K = = = 3.26 10 M DIF: L3 REF: p. 607 OBJ: 19.3.2 159. ANS: pH = –log [H ] = –log (6.8 10 ) = –(–6.2) = 6.2 or pH = –(log 6.8 + log 10 ) = –(0.833) – (–7) = 6.2 DIF: L2 160. ANS: 3 10 REF: p. 599 OBJ: 19.2.2 DIF: L2 161. ANS: REF: p. 607 OBJ: 19.3.2 K = = = = 0.000 000 02 = 2 DIF: L3 162. ANS: U He + DIF: L2 163. ANS: 8 years 10 REF: p. 607 OBJ: 19.3.2 REF: p. 800 OBJ: 25.2.1 Th STO: SC.A.2.4.3 DIF: L1 164. ANS: 0.63 g REF: p. 804 OBJ: 25.2.2 STO: SC.A.2.4.3 DIF: L3 STO: SC.A.2.4.3 165. ANS: 8 days REF: p. 804, p. 805 DIF: L3 166. ANS: 11,460 years ago REF: p. 804 OBJ: 25.2.2 STO: SC.A.2.4.3 DIF: L3 REF: p. 806 OBJ: 25.2.2 STO: SC.A.2.4.3 OBJ: 25.2.2