Check for Understanding#2
advertisement

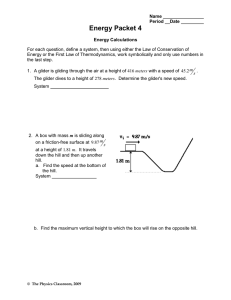
Check for Understanding #2 October 23/26 1. Identify: the parts of this chemical reaction: A + B AB A and B in this reaction are known as reactants, and AB is the product. 2. Diagram: the energy changes that can take place in a chemical reaction. Exothermic: energy from the reactant is higher than that of the product. Endothermic: energy is absorbed, so more energy is found in the product than the reactants. 3. Explain: why the number of atoms of reactants must equal the number of atoms of products formed. There must be an equal amount of energy on both sides of the equation. No energy is gained or lost. 4. Describe: the importance of enzymes to living organisms. Enzymes speed up the chemical reactions in the bodies of living organisms so they can survive. 5. For the following chemical reaction, label the reactants and products, and then balance the chemical equation. _H2O2__H20 + 02 reactants: H2O+O2, product: H2O2; 2H2O22H2O+O2 6. Draw a diagram of a roller coaster and write a paragraph relating the ride to activation energy and a chemical reaction. When a roller coaster begins up a hill, it gains potential energy. Once it starts down a hill, that energy is released as kinetic energy. The activation energy is needed just to get the roller coaster up the hill. Then energy continues to transfer from potential and kinetic and vice-versa.