File
advertisement

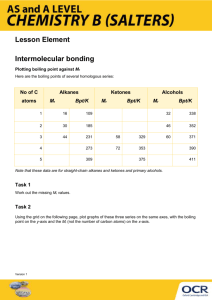
2006 (A) ANNOTATION: 6a) Glucose has all possibilities. Cyclohexane has nothing but London (default) b) Glucose has hydrogen bonds that can bond to water molecules well, thus dissolving well. Cyclohexane is the opposite. Also, glucose is polar. Cyclohexane is nonpolar, so it does not bond with h2o molecules as well to dissolve. c) i) 1—hydrogen and dipole dipole (H20 is involved!) (Intermolecular) 2—Covalent bonds within the molecule are broken (Intramolecular) ii) DUH NO. BOILING IS PHYSICAL CHANGE NOT CHEMICAL! AKA. InTERmolecular forces are broken. Water molecules itself are not separated into something different. GUIDELINES: 2006 (B) NONE 2007 (A) NONE 2007 (B) NONE 2008 (A) ANNOTATION: 6a) Pyridine is polar and can form hydrogen bonds. Benzene is nonpolar, cannot form hydrogen bond with water. Blablabla~ b) Dimethyl ether has London dispersion and dipole dipole (weak). Ethanol has those IMFs of dimethyl ether AND hydrogen bond that can be formed with water molecules, which are strong IMF which needs high bp to break the bonds during boiling. C) SO2: dipole dipole & London forces (weak!). Needs low mp to break bonds. SiO2: Network covalent, strong bonds (much stronger than intermolecular forces), needs high mp to break bonds. GUIDELINES 2008 (B) NONE 2009 (A) Question 6 ANNOTATION: a) I) LOOK AT SIZE if they IMFs are the same. H2S: more electrons, more polarizable than H20 therefore stronger London dispersion. ii) H2S: H—S bond is less polar than H—O bond (dipoledipole is all about polarity)—look at electronegativity between S and O (S<O)—so H2S has weaker d-d force. GUIDELINES 2009 (B) NONE 2010 (B) NONE 2010 (A) Question 5 ONLY d and f. OMIT e. ANOTATION: d) COURSE ITS FALSE! IT’S A TRICK. The bonds they tell you are INTRAMOLECULAR FORCES. BOILING is about breaking INTERmolecular forces. !!!! f) Ethanol has strong hydrogen bonds. Ethanethiol doesn’t. Ethanol can thus bond with water molecules and dissolve, but ethanethiol cannot. GUIDELINES 2011: (A) 5. Hydrazine is an inorganic compound with the formula N2H4 . The normal boiling point of N2H4 is 114°C, whereas the normal boiling point of C2H6 is −89°C. Explain, in terms of the intermolecular forces present in each liquid, why the boiling point of N2H4 is so much higher than that of C2H6. ANNOTATION N2H4: Has all 3 IMFs. C2H6 only has the weakest London. So it needs more energy to break the stronger bonds of N2H4, which comes in higher bp. GUIDELINES N2H4 is a polar molecule with London dispersion forces, dipole-dipole forces, and hydrogen bonding between molecules, whereas C2H6 is nonpolar and only has London dispersion forces between molecules. It takes more energy to overcome the stronger IMFs in hydrazine, resulting in a higher boiling point. 1 point is earned for correct reference to the two different types of IMFs. 1 point is earned for a valid explanation based on the relative strengths of the IMFs. 2011 (B) 6. Use principles of molecular structure, intermolecular forces, and kinetic molecular theory to answer the following questions. Ethyl methanoate, CH3CH2OCHO, is synthesized in the laboratory from ethanol, C2H5OH, and methanoic acid, HCOOH , as represented by the following equation. C2H5OH(l) + HCOOH(l) ƨ CH3CH2OCHO(l) + H2O(l) Draw the complete Lewis electron-dot diagrams of a methanoic acid molecule and a water molecule in an orientation that allows a hydrogen bond to form between them. ANNOTATION You see H2O, the easiest. So we identify water. Use your chapter 22 skills to recognize methanoic acid. Now, only water hydrogen bonds with other substances. So it muz be h-bonding. GUIDELINES (Hydrogen Bonding Between Methanoic Acid and Water) 1 point is earned for a diagram showing a reasonable orientation between a methanoic acid molecule and a water molecule.