ChemicalKinetics
advertisement

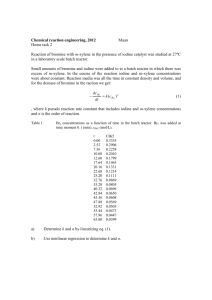
CHEMICAL KINETICS 1. Reaction Rate The rate of a chemical reaction is the change of concentration of a chemical species with time Rate 1 d[J] J dt where J is the stoichiometric coefficient of species J. A + 2B 3C + D Example: Rate 1 d[A] 1 dt or Rate 1 d[C] 3 dt Observe that reactant concentrations decrease with time (negative derivative) and product concentrations increase with time (positive derivative), use of the stoichiometric coefficient in the definition of rate insures that reaction rates are positive. Observe that use of the stoichiometric coefficient insures that the rate of a chemical reaction is the same regardless of which chemical species is used in the definition. In the above example, 1 molecule of A is consumed for every 3 molecules of C created; however, the reaction rates are equal due to inclusion of the stoichiometric coefficient. 2. Rate Law The rate law is an expression for the reaction rate in terms of concentrations of chemical species involved in the reaction. For example, Rate = k [A]m [B]n where k is a rate constant, m is reaction order with respect to A, n is the reaction order with respect to B, and m+n is the overall reaction order. The rate constant is independent of the concentrations of the chemical species involved in the reaction. However, it depends on other factors such as temperature or ionic strength, e.g., k(T). The units of the rate constant depend on the overall reaction order. The can be deduced by recognizing that Rate has units of concentration per time. Concentration is usually measured in molarity (M) for solution reactions and in molecules/cm3 for gas phase reactions. Example: d [A] k [A] [B] dt M kMM s k M 1 s 1 In general, the form of the rate law is NOT determined by the reaction stoichiometry, but is determined by the reaction mechanism. Example: Br2 + I2 2BrI which reacts by a one-step mechanism Example: d[Br2 ] k [Br2 ] [I 2 ] dt Br2 + H2 2HBr which reacts in a complicated mechanism in which the product, HBr, can react with a reactant, Br2. d [Br 2 ] k [H 2 ] [Br2 ]1/2 dt 1 k [HBr] / [Br 2 ] Rate laws are expressed as the differential of concentration with time, whereas experimental measurements are usually concentrations. Thus, in order to match theory with experiment, either the rate law must be integrated or the data must be differentiated. 3. Chemical Kinetics The goals of chemical kinetics are to: Experimentally measure rates of important chemical reactions to understand complex chemical systems. Current examples of important fields in chemical kinetics include: Combustion and atmospheric chemistry (gas phase). Biochemical pathways and polymer synthesis (liquid phase). Semiconductor and superconductor sythesis (solid phase). Experimentally determine rate laws, and suggest possible mechanisms that are consistent with the observed rate law. Measure dependence of rate constant on other experimental variables: Temperature: All reaction rates depend on temperature. Pressure: Gas phase reactions, e.g., O + O2 O3, depend on overall pressure. Changes in the stability of solid phases will lead to different reaction rates at different pressures. Ionic Strength: Electron and proton transfer rates of biochemical reactions depend on pH. Use kinetic data to characterize the energy and composition of the "transition state" in a chemical reaction. 4. Bromination of Acetone The reaction of bromine with acetone CH3COCH3 + Br2 CH3COCH2Br + Br- + H+ is known to be acid-catalyzed. Thus, the following form of the rate law is assumed d [Br2 ] k [CH 3 COCH 3 ] p [Br2 ]q [H ]r dt Bromine is a reddish-brown, and all the other reactants and products are clear. Thus, the reaction rate is conveniently measured by monitoring the concentration of bromine with a spectrophotometer. The form of the rate law can be simplified by arranging the experimental conditions so that the concentration of one species is much smaller than the others, a technique known as “flooding”. The concentration of the other species therefore remain essentially constant during the course of the reaction. In this case, [Br2] << [CH3COCH3], [H+]. Thus the rate law reduces to d [Br2 ] k obs [Br2 ]q dt where k obs k[CH 3 COCH3 ] p [H ] r The exponent q can be determined by measuring the concentration of bromine [Br2] as a function of time t and comparing the observed data to the integrated rate laws for various values of q: Order (q) Differential Rate Law Integrated Rate Law 0 d[Br2 ] k obs [Br2 ]0 k obs dt [Br2 ] [Br2 ]0 k obs t 1 d[Br2 ] k obs [Br2 ]1 dt ln[Br2 ] ln[Br2 ]0 k obs t 2 d [Br2 ] k obs [Br2 ]2 dt 1 1 k obs t [Br2 ] [Br2 ]0 The exponents p and r can be determined by concentration studies. The concentration of acetone [CH3COCH3] and acid [H+] are systematically varied The effect on kobs, which is proportional to the reaction rate, is observed. The rate constant k is then determined for each reaction run through the definition of kobs. 5. Reaction Mechanism A complex reaction can be written as a sequence of elementary reaction steps. The sum of the elementary steps equals the stoichiometry of the overall reaction. Example: AB I IB C A 2B C The rate law can also be deduced from the elementary steps. All the steps through the rate-determining step are important in determining the rate law. In the case where the first elementary step is also the rate determining step, then the observed rate law is simply the rate expression for that elementary step. The bromination of acetone is a reaction for which the first step is believed to be the rate limiting step. Thus, the form of the rate law predicts the first step of the reaction mechanism. It is generally true that the molecularity of the transition state can be determined from the form of the rate law. See Espenson, “Chemical Kinetics and Reaction Mechanism”, McGraw-Hill, 1981, p. 90 for a very readable discussion on this topic. Note that it is usually the case that the rate law is known from experiment and that the elementary steps must be postulated so as to be consistent with the observed rate law. It is for this reason that it is not possible to “prove” a mechanism using kinetics. Rather, reasonable mechanisms are put forward, and kinetic experiments are designed to distinguish among them. 6. Temperature Dependence The temperature dependence of the rate constant k(T) is often found to obey the Arrhenius equation k Ae Ea RT where A is the pre-exponential or frequency factor, Ea is the activation energy, and R is the gas constant. The values of A and Ea can be determined by linearizing the Arrhenius equation ln k Ea 1 ln A R T A plot of ln k versus 1/T will have a slope of -Ea/R and an intercept of ln A. ln A slope = -Ea/R ln (k) 1/T T increases In the collision theory of bi-molecular gas phase reactions, the frequency factor A is interpreted as the frequency of encounters among reactants irrespective of energy. The activation energy Ea is the minimum energy reactants must possess in order to form products. The Boltzmann distribution predicts that the exponential function in the Arrhenius equation is the fraction of collisions with energy greater than Ea. The product of the frequency factor and the Boltzmann exponential functions therefore gives the reaction rate constant. There is an analogous interpretation of these terms for reactions that occur in solution. In light of the collision theory interpretation, the activation energy Ea is also often interpreted as the energy of the transition state relative to the reactants. Collision theory is the most simple model of chemical kinetics. Reactions involving free radicals or stable intermediates are known to display Arrhenius plots with negative values of Ea! The interpretation of activation energy as the energy of the transition state is not applicable in these cases, as the actual kinetics is much more complex than the collision theory model. 7. Exercise Find a recent article in a chemistry journal that reports the results of a kinetics experiment. (You might browse through issues of “Journal of Chemical Physics” and “Journal of Physical Chemistry” in the library.) Use the article to answer the following questions. Report the authors, title, and full reference of the article. What chemical reaction is under study? What experimental technique is used to measure the reaction rate? What is the rate law for the reaction? What is the rate constant (with proper units)? Discuss why knowledge of the rate of this reaction is important or interesting.