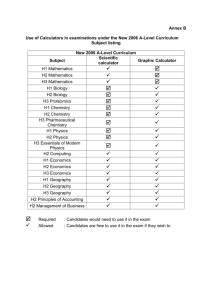
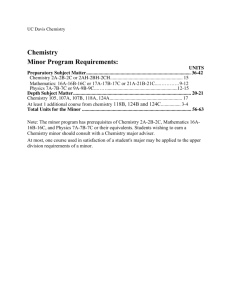
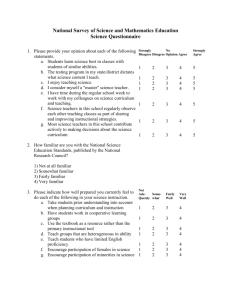
Stability and Instability in Complex Chemical Reaction Networks
advertisement

An Introduction to Chemical Reaction Network Theory Martin Feinberg Morrow Professor of Chemical and Biomolecular Engineering & Professor of Mathematics The Ohio State University In nature there are millions of distinct networks of chemical reactions that might present themselves for study at one time or another. Each new network, taken with kinetic rate functions for its various reactions, gives rise to its own system of differential equations for the species concentrations. These systems are almost always large and nonlinear (typically polynomial). It would appear, then, that chemistry should be a rich source of dynamical exotica. Yet there is a remarkable amount of stability in chemistry. Indeed, chemical engineers generally expect homogeneous isothermal reactors, even highly complex ones, to behave in quite dull ways. Although this tacit doctrine of stable behavior is supported by a long observational record, there are certainly instances of homogeneous isothermal reactors that give rise, for example, to bistability or even chaotic behavior. The vast landscape of chemical reaction networks, then, appears to have wide regions of intrinsic stability (regardless of parameter values) punctuated by smaller regions in which instability might be extant (for at least certain parameter values). Chemical reaction network theory is a rapidly developing part of mathematics that aims to understand that landscape in a coherent way – in particular, to draw firm connections between the structure of a reaction network and the variety of dynamics the network might admit. In a talk aimed largely at mathematicians who are inexpert in chemistry – but also at chemists and biochemists who are inexpert in modern mathematics – I will try to give examples of some recent and quite powerful results.