[12] MB Emelko, Removal of viable and inactivated
advertisement
![[12] MB Emelko, Removal of viable and inactivated](http://s3.studylib.net/store/data/008455123_1-3af44e46ce1295c0cac0a6e37788609b-768x994.png)
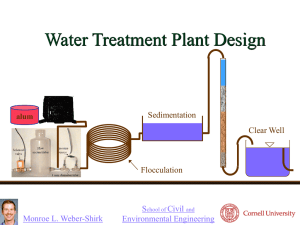
Mervyn Besra Illumin Article Writ 340 About the Author: Mervyn Besra is a third year Civil and Environmental Engineering student at the University of Southern California. Contact Email: Besra@usc.edu Keywords: Civil Engineering, Water Treatment, Health, Engineering Design Safe to Drink: The Engineering Science of Water Remediation [The water used everyday by billions of people worldwide is treated through an intricate process before it can safely reach its consumers. However, this treatment process, seldom known to most people, people did not happen overnight. Throughout centuries civilizations have come up with various methods to treat water, many of which are still in use today. This article will attempt to outline the process of an actual water treatment facility in the United States while also concluding with a discussion about its importance and future.] Water, the essential component of life; each day, billions of gallons of water are pumped to people across the world for consumption, cleaning, and recreation. It flows in what seems like unlimited supply from faucets, showerheads and sprinklers all around. But have you ever wondered where it all comes from and the steps that are taken to make sure that it’s safe to use? Contrary to most notions, our water supply is not as vast as it appears to be. 96% of the water on earth is located in the ocean and is undrinkable due to the heavy salinity in these bodies. Out of the remaining 4% of freshwater on the planet, only 0.04% is available to us and must go through an extremely intricate process of treatment before it can become useable [1]. Most of the water that comes out of our faucets is siphoned from either surface water such as lakes, rivers, or streams or ground water taken from aquifers existing in caverns below the earth’s surface. From here it goes through a surface water treatment facility that is comprised of numerous components, which treat the water at different stages[1][3]. This article will attempt to explain what goes on during the many stages of treatment. History The concept of water treatment goes back several millennia where sources as early as 2,000 BC discuss the use of water treatment within their societies. Among these early pioneers were those living in Greece and India who came up with creative methods to purify water through boiling and straining [2]. Due to an inadequate knowledge of pathology, the main motivation for water treatment at the time was taste instead of sterility. As a result, these systems focused primarily on treating turbidity, or cloudiness in water and sometimes fell short at producing water that was contaminant free [2]. In 300-200 BC, Rome pioneered one of the most intricate water systems of the ancient world, the aqueduct. This system used a complex array of underground pipes to supply water to a city from far distances using only gravity. The Roman idea of an aqueduct was so successful that it can still be found in numerous European countries today such as France, Germany, and Spain. Even the United States has considered implementing some ideas found in the aqueduct system to further improve its water infrastructure[3]. The birth of the modern concept of water engineering occurred in 1676 with the invention of the microscope. After its creation by Van Leeuwenhoek, people were able to observe microorganisms in water and eventually speculated that some of them were responsible for causing disease. This idea was further reinforced after a cholera outbreak occurred in 1854 causing numerous fatalities and even more complications. John Snow, a British scientist found that the direct cause of the outbreak came as a result of water contamination and successfully experimented with chlorine to disinfect the water [2][3]. This discovery sparked the notion that taste and turbidity alone were not enough to determine acceptable water quality, which eventually lead to numerous countries adopting a modern municipal water treatment system [3]. Modern Water Treatment in Developed Countries In most developed countries, water suitable for drinking passes through a facility designed to treat the water at several different stages. Though the details of the components may vary slightly depending on location and source, a typical treatment facility will include most of the following parts and processes, a pre-sedimentation basin, rapid-mix tank, flocculation tank, sedimentation tank, filtration, and disinfection[7][9]. Figure 1: General components of a surface water treatment plant [7]. Pre-sedimentation Basin Before going through the actual treatment processes, surface water siphoned from either a river or a lake must enter a large reservoir known as a pre-sedimentation basin, where it is detained for about 8 hours. During this time, larger contaminants and solids such as sand, silt, gravel, and larger debris settle at the bottom and are manually removed after the settling period. This accomplishes two purposes. It prevents these larger particles from potentially clogging up or interfering with the processes in subsequent tanks and it serves as the first stage in treating the water for turbidity. After this stage, the surface water can then be pre-chlorinated and pumped into the actual surface water treatment plant[10]. Rapid Mix Tank The first stage in actual treatment, occurs in a chamber called a Rapid Mix Tank. The tank, usually around 60-75 cubic ft in volume, treats water using a method called coagulation. This is the process by which smaller particles are combined to form larger particles called aggregates in an effort to make the collection of those particles easier to remove [8]. The process is similar to that of a snowball rolling down a hill that gets larger as it collects more snow particles in its path. Figure 2: Description of the coagulation mechanism [8] The coagulation process in this tank is accomplished by rapidly mixing a positively charged ionic compound such as iron chloride (FeCl3) into the water using a propeller [9]. When this is done, the negatively charged clay and silt particles still remaining, combine with the positively charged ionic compound to form larger particles. This process is usually accomplished in about a minute or less, after which the water is then transferred to the flocculation tank [8]. Figure 3: Rapid Mixer and Flocculation Tank schematic. [8] Flocculation Tank Also known as a slow mix tank, the typical flocculation tank is composed of three smaller sub tanks connected in series. Each individual tank, containing its own propeller continues to mix the water at varying speeds. Of the three, the first chamber mixes the water at a faster rate than the others for the purpose of stimulating more coagulation between the impurities and ionic compounds[8]. In the second compartment the speed of the propeller is reduced allowing for the coagulated particles to combine with other particles to form floc, a conglomerate of bacteria and particulate impurities. During this stage, the charges on the particles themselves become neutralized as bigger clumps of material cleave to each other [8]. The final chamber allows the process of flocculation to continue by reducing the propeller speed even further. By this time most of the particles in the water have been neutralized and the slow speed allows for bigger chunks of floc to form without being cut by the spinning propeller blades. As this occurs the chunks of floc become heavier and start to sink when not agitated by the moving propellers[8][9]. Sedimentation Tank Like the pre-sedimentation tank before it, the purpose of the sedimentation tank is to treat turbidity by allowing suspended particles to settle at the bottom as a result of gravity. After going through the various mixing tanks, the water now contains an almost suspended layer of floc that has now been neutralized. Within a matter of two to four hours, the now accumulated chunks of particulates fall to the bottom where they are eventually removed. This stage of the process removes roughly 90% of the contaminants, which also include bacteria. Sedimentation also prevents excess clogging of the filters during the next stage of treatment [6]. Figure 3: Mechanism of a sedimentation tank. [6] Filtration Before leaving to the distribution system, the water is taken through one final step, which not only treats remaining turbidity but also makes sure that infectious microorganisms are thoroughly removed through the process of filtration. Numerous methods of filtration exist for treating water such as slow sand and rapid sand filtration, both with their inherent pros and cons. However, one of the most efficient filters used today is the dual media filter, which uses a cylindrical tank containing a top layer of anthracite, a hard form of coal. A layer of sand beneath the anthracite follows this, which is then supported underneath by gravel, which keeps everything in place [5]. Dual media filtration works by using each medium to remedy a specific problem with the water. The top layer of anthracite is responsible for treating taking care of odor and color problems still present in the water; furthermore, it is also effective at neutralizing mild toxins that are sometimes present. The bottom layer of sand then takes care of the remaining suspended particles leaving the final product 99.9% free of contaminants [5] [9]. Aside from the benefits of high filtration efficiency, dual media filters offer other benefits such as automatic back washing, a process used for cleaning out filters by pumping water from the reverse end and cleaning out the trapped particulates. This feature prolongs the life of the filter and allows it to be used for longer periods of time before it has to be regenerated. The filtered water is then given a final dose of chlorine treatment, after which it is transported to a distribution system for human use [5][7]. Figure 4: Dual Media Filter [5] Current Problems and the Future of Water Treatment While it may true that implementing modern day water treatment systems into our infrastructure has greatly benefited us in numerous ways, they are still far from perfect as numerous cases of water borne epidemics have surfaced despite the inception of these facilities. One specifically noteworthy case was an incident in Milwaukee WI involving a massive outbreak of a pathogen known as cryptosporidium. The organism, a single celled protozoa, was immune to chlorine decontamination and managed to slip through the typical filtering system used during that time. The result was a severe epidemic, which infected 403,000 residents and caused at least 104 deaths making it the largest waterborne disease outbreak documented in the United States [4]. Outbreaks such as these have forced scientist and engineers to continuously come up with innovative solutions to better improve water remediation systems. This is done by analyzing potential flaws in current designs, and researching new methods to treat specific types of pathogens. For example, in the case of Milwaukee, it was discovered that the pathogen was both immune to chlorination and small enough to fit through the crevices of sand in the filter [4]. As a result scientists researched other methods that would work and found that ozone (O3) and ultra violet radiation killed cryptosporidium [11]. Also, as a result of the outbreak new methods of filtration were tested including the current dual media filter now used in many parts of the US. Conclusion Water quality engineering is a science that many of us take for granted. Perhaps the fact that many don’t seem to notice is a good indicator of how far we have succeeded in providing adequate treatment systems, supplying countless gallons of consumable water to communities and cities worldwide. Although few will argue that much can still be done to ensure that our methods become better with time, most will agree when looking back that we have come a long way in developing intricate and fascinating methods to ensure that our water is safe to drink. Works Cited [1] G. Tyler Miller & Scott Spoolman, Environmental Science, Brooks/Cole, 2010 [2] Baker M.N., Taras M.J., 1981, The quest for pure water – The history of the twentieth century, volume I and II, Denver: AWWA [3] S.M. Enzler, History of Water treatment, http://www.lenntech.com/history-watertreatment.htm [4] N J Hoxie, J P Davis, J M Vergeront, R D Nashold, and K A Blair, Am J Public Health. 1997 December; 87 [5] Jennifer Peters Dodd & James D. Fettig, Filter Backwashing, http://www.cee.vt.edu/ewr/environmental/teach/wtprimer/backwash/backwash.html [6] Mountain Empire Community College, Sedimentation Basin, http://water.me.vccs.edu/concepts/basin.htm [7] APEC, How do Water Treatment Plants Work, http://freedrinkingwater.com/water_quality/quality1/1-how-do-water-treatment-plantswork.htm [8] Mountain Empire Community College, Coagulation and Flocculation http://water.me.vccs.edu/courses/env110/lesson4.htm [9] EPA Guidance Manual, Turbidity Provisions, April 1999 [10] City of Witchita, Aquifer Storage and Recovery, Pre-sedimentation Basin, 2011 [11] Mac Kenzie WR, Hoxie NJ, Proctor ME, Gradus MS, Blair KA, Peterson DE, Kazmierczak JJ, Addiss DG, Fox KR, Rose JB, et al. A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. N Engl J Med. 1994 Jul 21 [12] M.B. Emelko, Removal of viable and inactivated Cryptosporidium by dual- and trimedia filtration, Water Res (University of Waterloo) Issue 37 July 2003