AGREEMENT BETWEEN - Cancer Data Registry of Idaho
advertisement

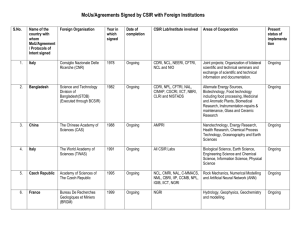
AGREEMENT BETWEEN applicant AND CANCER DATA REGISTRY OF IDAHO applicant AND CANCER DATA REGISTRY OF IDAHO PURPOSE OF AGREEMENT The Cancer Data Registry of Idaho (CDRI) is a population-based cancer registry that collects incidence and survival data on all cancer patients who reside in the state of Idaho or who are diagnosed and/or treated for cancer in the state of Idaho. This document serves as an agreement between CDRI and applicant. The purpose of this agreement is to secure the confidential exchange of data from CDRI to applicant to be used in a composite database for research, statistical or public health purposes as permitted by Idaho Department of Health and Welfare Rules and Regulations, Title 5, Chapter 1, "Rules Governing the Protection and Disclosure of Department Records," and §57-1706 of Idaho Code. In the event that information which may identify patients or report sources is needed for research, statistical or public health purposes, the following policies will be practiced. POLICIES ON DATA DISCLOSURE REQUEST FOR CONFIDENTIAL DATA Investigators seeking approval to access confidential data from CDRI must submit their proposal in writing using the form contained herein. Comprehensive research protocols should be attached. Applications will be reviewed and evaluated by the Cancer Analysis Work Group (CAWG), which consists of individuals from the Division of Health, Idaho Department of Health and Welfare, and the Cancer Data Registry of Idaho, Idaho Hospital Association. REQUEST FOR HOSPITAL SPECIFIC INFORMATION Researchers requesting data containing hospital identifiers must contact CDRI directly for the information. The data will be released only upon written approval by the source hospital(s) stating that the facility agrees to participate in the study. All research protocols and a copy of the proposal will be sent to the facility's administrator and/or institutional review board for approval. PATIENT CONTACT Researchers proposing to make contact with patients or family members of patients must contact CDRI directly, not the patients or family members. CDRI will contact physicians to identify patients who should not be contacted. CDRI will assume passive approval by physicians who do not respond by a specific date. In some cases, CDRI may contact the patient directly for his/her permission to participate in a proposed project. CONDITIONS OF AGREEMENT This agreement prohibits the following: 1. The use of data for other than the stated purposes. 2. Disclosure by applicant to any party or organization other than those listed within this agreement unless otherwise authorized. 3. The release of death certificate information without prior approval from the Idaho Bureau of Vital Records and Health Statistics. 4. The transfer of confidential data via the Internet, unless there is a plan for data encryption. This agreement requires the following: 1. Destruction of data (and copies) after the stated purposes have been fulfilled. 2. Recipients of CDRI data accept all liability and legal expenses which may accrue to CDRI resulting from an unauthorized re-disclosure of confidential data by the investigator or associated staff. 3. A violation of the signed confidentiality pledge will result in a material breach of contract and can result in appropriate disciplinary actions, civil damages, and criminal prosecution under Idaho laws. 4. Any changes in proposed methodology as described in this agreement require a written request for amendment and CDRI authorization. 5. All manuscripts for publication that make reference to CDRI, or use CDRI data, will be subject to review by CDRI and the Cancer Analysis Work Group. CDRI shall be provided courtesy copies of all final publications. 6. Signed confidentiality pledges from persons having access to CDRI data as specified within this contract, and other approved projects, will be sent to CDRI prior to release of data by CDRI. 7. Requests for data linkage projects are usually carried out by CDRI staff. However, if a researcher obtains permission from CDRI to conduct a linkage project, records from CDRI that do not link with the secondary database will be discarded immediately and will not be available for use. Special permission can be obtained from CDRI to retain unlinked data that has been de-identified. Researchers are required to report the number and percentage of cases that were linked, and return to CDRI a signed statement verifying that unlinked cases were discarded and/or de-identified, using the form contained herein. Case data are considered de-identified when the following variables have been stripped from the case: full name, zip code, county code, Social security number, birth day, birth month. 8. Compliance with applicant's internal policies as specified in item F of this application. 9. CDRI’s checklist of requirements shall be attached to all proposals submitted to CDRI. PROVISION FOR TERMINATION OF AGREEMENT If at any time CDRI wishes to discontinue participation as a data contributor, this agreement can be terminated without liability. CANCER DATA REGISTRY OF IDAHO CHECKLIST This form must be completed and submitted with each proposal to use data from the Cancer Data Registry of Idaho (CDRI). This is to assure that pertinent information has been addressed and included with the proposal. Type of proposal submitted: 1) New 2) Amended 3) Annual review YES 1. Has the project received IRB approval? If so, please attach to proposal. 2. Has a copy of CDRI’s policies regarding patient contact been given to the principal investigator? 3. If invasive testing is planned, a sample patient consent form should be attached. 4. Attached are signed confidentiality pledges, as required on page 4 of the agreement between CDRI and applicant. 5. If data linkage with a secondary database is proposed, a plan for data security is attached. 6. If patient-identifying data are transmitted via Internet, a plan for data encryption is attached. NO NOT Applicable APPLICATION TO ACCESS CONFIDENTIAL DATA A. Name of researcher responsible for conducting the study: B. Affiliation: C. Specific data items requested and desired format: D. Purpose and intent of requested data. List all uses of the CDRI data: E. List all persons who will have access to the data and their positions. Principal investigator is required to sign CDRI's confidentiality pledge and is responsible for assuring that all persons listed below are aware of the conditions of agreement. Persons not listed below will not be authorized to access confidential data. F. Describe policies and procedures that will be used to protect confidentiality of data once released to your possession. G. Describe the methodology of the research including plans to conduct follow-backs and direct contact with patients and/or family members, physicians, and hospitals. Attach research protocols, if any. CANCER DATA REGISTRY OF IDAHO CONFIDENTIALITY PLEDGE The contract signatory for applicant and all persons having access to the CDRI data must agree with and affix their signature to the following: As a condition for my access to confidential CDRI data, I agree to uphold the confidentiality of the data in accordance with the following requirements: 1. I will avoid any action that will provide confidential information to unauthorized individuals or agencies. 2. I will not make copies of or view any CDRI records or files to which I do not have specific authorization. 3. All confidential data to which I have access will be maintained in a safe manner which restricts unauthorized individuals from access. 4. I will not discuss information that might lead to identification of individuals described in CDRI’s files with any unauthorized person. 5. I will limit my use of confidential CDRI data to the purposes for which I have been specifically authorized. 6. I will not give my password(s) or file access codes to any unauthorized person, and upon termination of the project will arrange for appropriate disposition of all confidential data in my possession. 7. If I become aware of unauthorized access to, or divulgence of confidential CDRI data by someone else, I will report it immediately to the Cancer Data Registry of Idaho Vice President, Operations and Registry Services. I understand that failure to report violations of confidentiality by others is just as serious as my own violation and may subject me to legal prosecution. I understand that if I fail to keep my pledge of confidentiality I will be denied access to the CDRI data and I may be subject to legal penalties. I hereby swear or affirm to comply with CDRI policies for data disclosure, the delineated conditions of this agreement, the provision for termination of this agreement, and the assertions within the application completed by applicant. I will effectively communicate all provisions of the agreement between CDRI and applicant to all persons who have access to CDRI data. I will assure that all persons at applicant having access to CDRI data, as well as researchers using the database, will sign confidentiality pledges. Signature Date FOR OFFICE USE: Response to requesting party: Application for requested data was: accepted denied Reason for denial: Comments: Vice President, Operations and Registry Services Date