Specific Heat Capacity Lab: Physics Experiment
advertisement

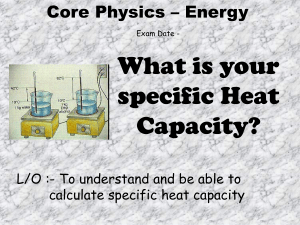
IB Physics Determining Specific Heat Capacity You have learned that the specific heat capacity of a material can be defined as… … the amount of heat energy needed to raise the temperature of one kilogram of a material through one Kelvin. Specific heat capacity is measured in joules per Kilogram per Kelvin = J Kg-1K-1 specific heat capacity = c= Experiment: heat energy transferred mass x change in temperature ∆Q m ∆T or ∆Q = m c ∆T To determine the specific heat capacity of aluminium, copper and water. Use an electrical immersion heater to heat a sample of aluminium, copper and water, each for 3 minutes. Take measurements that allow you to determine the specific heat capacity of each material. Q1. How will you measure… a. mass? b. change in temperature? c. heat energy transferred? Diagram: Draw the experimental arrangement for… a. a solid b. water. Q2. How will you plan to minimise experimental error for … a. Both experiments (give three methods)? b. The water experiment (give one more method)? Q3. State one further source of error in the water experiment. Results: Record your readings and determine ∆T, ∆Q and the specific heat capacities: Material Aluminium Copper Water m / kg T1 / K-1 T2 / K-1 ∆T / K I/A V/V t/s ∆Q / J c / J Kg-1K-1 Q4. Which material heats up the ‘slowest’? Q5. Which material has the greatest specific heat capacity? Approximately the same amount of energy is needed to raise the temperature of any molecule of any material by one Kelvin. Q6. Out of the copper and aluminium, which both had the same mass, and which both received the same total amount of energy, which material must have the most molecules? Why? Extension 1: Consider the water experiment. Given that the specific heat capacity for glass is about 700 J Kg-1K-1 and that the glass is heated over the same temperature range (T1 to T2) as the water, how could you determine a more accurate value for cwater? Extension 2: If you had more time, how could you take measurements and use a graphical method to find c for a material? (Hint: Think of the formula ∆Q = m c ∆T)