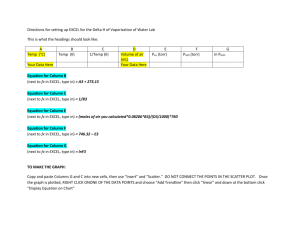
biology department - The University of the South
advertisement

1 BIOLOGY DEPARTMENT STUDENT SURVIVAL GUIDE Fall 2006 Compiled by the Members of The Biology Department at The University of the South Fall 2006 2 Table of Contents CHAPTER 1: LAB POLICIES ----------------------------------------------------------------------3 CHAPTER 2: USING YOUR SEWANEE ANGELS ACCOUNT----------------------------6 CHAPTER 3: LABORATORY NOTEBOOKS--------------------------------------------------8 CHAPTER 4: RESEARCH PROPOSAL GUIDELINES ------------------------------------ 11 CHAPTER 5: A GUIDE TO WRITING SCIENTIFIC PAPERS -------------------------- 27 CHAPTER 6: LITERATURE CITED ----------------------------------------------------------- 33 CHAPTER 7: AN INTRODUCTION TO GRAPHS AND DATA SUMMARIES ------ 36 CHAPTER 8: AN INTRODUCTION TO MICROSOFT EXCEL 2000 ------------------ 45 CHAPTER 9: USING EXCEL TO PLOT DATA (BIOLOGY 132) ----------------------- 65 CHAPTER 10: INFERENTIAL STATISTICS OR "STATISTICAL TESTS" --------- 71 CHAPTER 11: USE OF OXFORD® DIGITAL DIAL PIPETS ---------------------------- 83 CHAPTER 12: SPECTROPHOTOMETRY---------------------------------------------------- 87 CHAPTER 13: OSMOMETRY ------------------------------------------------------------------- 89 CHAPTER 14: USING MICROSCOPES ------------------------------------------------------- 91 CHAPTER 15: USING FIRST SEARCH AND OTHER LIBRARY RESOURCES --- 93 3 CHAPTER 1: LAB POLICIES APPLICATION OF THE HONOR CODE TO BIOLOGY LABORATORY Students are to pay scrupulous attention to the Honor Code when making entries in their laboratory notebooks and writing proposals and papers. Make the following guidelines your own: 9 All data and observations in my notebook are to the best of my knowledge accurate reports. Fabrication and alteration of data are unauthorized. 9 Copying data from another student’s book is unauthorized unless we obtained the data together. Reading any answers to questions or discussions in another student’s book or allowing someone to read my book are unauthorized, whether or not we were partners. 9 Calculations and discussions may be written outside the laboratory. These may be discussed with others, but the actual calculations and discussions in my book must be written by me. I must state clearly which of these were influenced or aided by another student or instructor other than my own. Unless your professor indicates otherwise by asking specifically, in writing, for group write-ups and proposals, this rule also applies to written material such as proposals and reports to be turned in and graded. These MUST be written individually. In the case of proposals, discussion within a lab group of the experiments to be run is highly recommended. Discussion facilitates understanding. However, proposals MUST be written individually. If there is more than one idea in a group regarding what to do in a second week of experimentation, individuals in a group may propose different experiments. However, during the second in-lab session all members of a group will be expected to run the same experiment. With regard to lab reports, while all the members of a group will be reporting on the same experiments and reporting the same data, these MUST be written individually. Again, you are encouraged to discuss the meanings of results but you are not allowed to write or use portions of each others' reports. 9 Entering data into the computer under any other than my name and ID number is unauthorized. In addition, proper citation of sources used in writing proposals and papers is a MUST. Use proper citation methods and formats as noted in the Survival Guide, using ideas and citing the source for these ideas but do not copy. 9 Never cut and paste from a web site into your paper 9 Never copy portions from any source without using quotations. 4 EQUIPMENT At times, students will check out equipment as it pertains to individual labs. Some equipment such as digital cameras and associated accessories can cost in excess of $300. It is the policy of the Biology Department that students will be held personally responsible for returning the equipment in working order or providing for its replacement. In the case of a group of students checking out equipment, all members of the group will be held equally responsible. LABORATORY SAFETY GUIDELINES Protective Equipment √ Wear appropriate Personal Protective Equipment (PPE) as instructed. This includes, but is not limited to, the use of aprons, goggles and gloves. In general, use this equipment whenever dealing with chemicals of particular hazard, or in large volumes or high concentration; or when working with flames, or gases under pressure. √ Do not wear PPE outside of the lab unless necessary (ie to the restroom or classroom). Doing so exposes other people and places to contaminants that should remain in the lab and vice verse exposing your experiment to outside contaminants. Keep this in mind when you must wear PPE to transfer work to another lab for example. √ Learn the location and operation of eyewash, safety shower, and fire extinguisher. Know what to do or whom to contact in case of an emergency. Personal Hygiene √ While Class Dress is not required, lab dress has its own requirements. Appropriate lab dress is as follows: Wear shoes that will cover and protect the whole foot, leather is best. Clothing, made of natural fibers, should cover the entire body; shirts should cover the entire torso. Long sleeves and long pants are best, however, sleeves should not be loose and floppy. You will not be allowed into lab without appropriate clothing. This is for your safety and the safety of those around you. √ Wash hands frequently: before you begin your experiment, when you change gloves, and before you leave lab. √ Tie back long hair to keep it away from flames and chemicals. √ Never smoke, eat, drink, or apply cosmetics in the laboratory area. Avoid touching your face, chewing on pens or other similar behaviors. √ Never taste a chemical. Check odors, only if instructed to do so, by wafting with one hand towards your nose. Behavior √ Act in a responsible manner at all times. No horseplay or fooling around should occur in the lab or experimental area. Never remove chemicals from the laboratory area without proper authorization. 5 √ Never perform unauthorized work, preparations, or experiments. √ Turn off the Bunsen burner when it is not in use. Never leave a flame unattended. √ Report any injuries to the professor or lab assistant, and seek medical attention immediately if needed. √ Keep walkways and work areas free from obstructions. Coats, book bags or other materials not directly needed to perform labs should be kept in lockers, in the hallway, or in another room not in use. Keep stools under benches when not in use. Chemical Hygiene √ Follow all disposal methods for chemicals as specified by professor or lab assistant. √ Treat all chemicals with the respect they warrant. √ In case of spill, immediately contact the professor or lab assistant. √ Wash off chemicals splashed or spilled on your skin or body immediately and thoroughly, removing contaminated clothing. Have your lab partner notify the professor or lab assistant immediately. √ Clean your lab bench, place all equipment and reagents on the bench as you found them, and wash your hands with soap and water at the end of each laboratory session. √ Dispose of all hazardous, Biohazardous, Radioactive and Hazardous Materials, as directed by your instructor, i.e. blood or bacteria in the “BIOHAZARD” bag, needles in the “sharps” container, and broken glass in “broken glass” container. There may also be waste containers for certain chemicals. When in doubt, ask. Biological Safety √ Practice Universal Precautions when appropriate. This means that you should assume that all biological materials are potentially infectious and take necessary precautions to prevent exposure. Field Safety √ Wear appropriate clothing to protect you in the out of doors. Sturdy shoes that cover your entire foot, long pants, and sleeved shirts are best. Dress for the weather, most of our labs will be in the field. Wear a hat and sunscreen if needed. √ Be aware that you are a visitor in the homes of various organisms: especially ticks, bees, poison ivy, and snakes. Your instructor will orient you to the habits of these organisms in our area. √ Please let your instructor know about any pertinent medical history you may have (ex. diabetes, anaphylaxis, colorblindness). This allows lab staff to be better prepared should the situation arise. 6 CHAPTER 2: USING YOUR SEWANEE ANGELS ACCOUNT Saving your working files to the Sewanee Angels server All Sewanee students are provided with a folder and disk space on the Sewanee Angels server of the university computer system. You can use this space to save important work such as term papers, class data, and your personal web page. The advantage of saving your work to this folder is that you can access it from any computer that is hooked up to the university computer system (e.g. in the library, in a classroom, in your dorm room) The navigational instructions to access your Sewanee Angels folder differs slightly, depending upon whether you are using a Mac or a PC computer: If you have trouble accessing your account, contact the Computing and Network Services help desk (x1369) or the Academic Technology Center help desk (1362). Connection Instructions for Macintosh Computers 1. 2. 3. 4. Click on ‘GO’. Type angels.sewanee.edu You will be prompted to give Username and Password. Type in your username (the same as your email username) and your password (the same as your email password), then click "OK". 5. A window will appear with a bunch of folders, one of which has your username on it. Double click on your folder to view the contents. Connection Instructions for PC Computers 1. 2. 3. 4. Click on the Start button in the lower left corner of the computer screen and select “Run” Type in \\angels.sewanee.edu and click “OK” A window will appear with a prompt for your Username and Password Type in your username (the same as your email username) and your password (the same as your email password), then click "OK". 5. A window will appear with a bunch of folders, one of which has your username on it. Double click on your folder to view the contents. Adding files to your Sewanee Angels folder To add files to your folder, do one of the following a. With your Sewanee Angels folder displayed on the computer, simply drag the file you have saved on your computer into your Sewanee Angels folder. b. Do a "Save as" command from whatever application you are using and from the Save window navigate to your folder before hitting the SAVE key Retrieving and using files from your Sewanee Angels folder To retrieve something from your folder 7 a. Connect to your Sewanee Angles folder (Mac—In the Chooser select Appleshare, select Appletalk Zone Sewanee Net Servers, select Sewanee Angels file server, enter your username and password; PC—In the Start/Run menu type \\angels.sewanee.edu). b. Drag the file you are interested in using onto the desktop of the computer you are working on. DON'T WORK ON A DOCUMENT DIRECTLY FROM THE SEWANEE ANGELS SERVER. Why? If the network connection goes down, you will lose any unsaved changes. Also, the computer will be slower if it has to work over the network. c. Save your modified work back to the Sewanee Angels folder when you are finished. 8 CHAPTER 3: LABORATORY NOTEBOOKS Following are a few hints for keeping your weekly laboratory notebook: Purpose State succinctly the goals of the exercise. If you are not sure of the purpose before you come to lab, leave a few lines for this section, then fill in the purpose when you are confident. Lecture Notes You may take lecture notes in your notebook or on other sheets of paper - whichever you prefer. The lecture notes will pertain to the lab you are doing and future assignments that relate to the lab. We would suggest that you take lecture notes in your lab notebook so that you do not have to keep track of separate sheets that could easily be lost. If you feel that some of the notes would be helpful for you to have in your possession while your lab notebook is being graded you should photocopy them. Procedures and Results These two can usually be combined in the laboratory record. Most of your procedures can be presented as a table or protocol that shows how you proceeded. A brief narrative is usually required to supplement a table, but this should include only sufficient information for a scientifically literate person to repeat your experiment. Do not include details like the tube size used, but do include the amounts of solutions mixed and their final concentrations. Don’t describe how to use a spectrophotometer or a balance, but do indicate that you used a Hach DR2500 scanning spectrophotometer to measure absorbance and an electronic top-loading balance to weigh chemicals. If you perform calculations, you should show one sample of each kind of calculation. Also make sure that you include units for parameters with units in the equation. Make sure, especially, that the correct unit accompanies your final calculation. Results can be interspersed with the procedures. If you do one portion of the lab and write those procedures, you may follow that with the results for that section before moving on to write the procedures for the next segment of the lab. If you find that you must include additional material for a procedure but have no space for it, indicate clearly the page where the additional material can be found. Each table and each graph (called figures) must be formatted properly. All tables should have a number and title above it (Table 1. Absorbance for pigment A at each wavelength tested.; see Table 1 below). Units should be found in column titles [Wavelength (nm)]. Any additional information should be in a caption found below the table. Graphs should also have a number and title, found below graphs as a caption (Figure 1. Absorption spectrum for pigment A.) Additional information, such as what each type of symbol or color of line means should also be in a legend to the figure. In addition, each axis must have a label, and most parameters will have units. There are many examples of properly labeled tables and figures found throughout the biology department’s lab manuals. 9 Table 1. Absorbance for pigment A at each wavelength tested. Wavelength (nm) Absorbance 420 0.12 440 0.34 460 0.55 480 0.22 Answers to problems or questions that appear in the text of the lab The answers may be reported at the end of the procedures and results section or in the body of the procedure and results section if you prefer. Many of the problems or questions in this section are presented in order to get you to make the proper conclusions about the day’s work. For that reason, some will seem redundant when you write your conclusions. Conclusions This is a very important part of your notebook. It is the place where you make your important observations, and can discuss possible errors or anomalous results. For example, in the photosynthetic pigment lab, identify each of the pigments you isolated and give your basis for these conclusions. 11 CHAPTER 4: RESEARCH PROPOSAL GUIDELINES A primary objective of introductory biology labs is to introduce you to, or enrich your understanding of, the scientific method. At various times throughout the semester, you will be testing hypotheses by conducting experiments or studies of your own design. These exercises give you a chance to be creative. At the same time, they require some advanced planning on your part. Prior to conducting your own study, therefore, you will be required to turn in a research proposal that justifies and describes your proposed research. This writing exercise helps you “think through” your experiment, and the proposal itself can be useful when writing your final lab report because you will have already begun writing some sections that will be included in your final report. The guidelines provided here are to help you prepare a research proposal. Note that while these guidelines are to be used for Biology 131/132 proposals, the format for upper level Biology courses may vary slightly, according to preferences of individual professors. Thus, it is recommended that you consult your professor for the appropriate proposal format for that upper level course. This chapter is presented in three sections, Proposal Format for Biology 131, Proposal Format for Biology 132, and a Sample Proposal. The Proposal Format is presented twice in order to convey inherent differences between field studies and lab studies; the first presentation is with Biology 131 examples, and the second with Biology 132 examples Research Proposal Guidelines: 131 Perspective A primary objective of introductory biology labs is to introduce you to, or enrich your understanding of, the scientific method. At various times throughout the semester, you will be testing hypotheses by conducting experiments or studies of your own design. These exercises give you a chance to be creative. At the same time, they require some advanced planning on your part. Prior to conducting your own study, therefore, you will be required to turn in a research proposal that justifies and describes your proposed research. This writing exercise helps you “think through” your experiment, and the proposal itself can be useful when writing your final lab report because you will have already begun writing some sections that will be included in your final report. The guidelines provided here are to help you prepare a research proposal. PROPOSAL FORMAT • All sections in your proposal, outlined below, should be identified with corresponding headings. • Use FUTURE TENSE consistently throughout the text when referring to your proposed research, as you are writing about activities that you will conduct in the future. Note that this is in contrast to your lab report, in which you will use the PAST tense, because your research will be complete when you write the formal report. • If you are working in a group, use first person plural (we), otherwise use first person singular (I). E.g. We/I will count the number of flowers produced at the end of an eight 12 week growing period. The passive voice may also be used. E.g. The number of flowers produced will be counted at the end of an eight week growing period. • All text within your proposal, including the methods, should be written in concise, but complete, sentences and organized into paragraphs. • When providing the scientific name of a plant or animal for the first time, remember to use upper case (capitalize) the first letter of the genus (the species remains in lower case), and either underline or italicize both the genus and species. After the first citation, you may simply refer to the common name, or the abbreviated scientific name. For example, “We propose to examine floral structures of cardinal flowers (Lobelia cardinalis)”. Thereafter you can just refer to the organism as the cardinal flower, or as L. cardinalis. • Number each page and staple or paper clip the pages together, before you hand your proposal. Check with your instructor on their policy/preferences for line spacing and printing on the blank side of used paper. • Refer to the Sample Research Proposal for an example that demonstrates the desired format, writing style, and approximate text length. • The format for research proposals outlined in the Student Survival Guide will not be used in this course. Instead, follow the formatting we provide to you. PROPOSAL CONTENT The proposal does not have to be long, but it should be complete, and include all of the following components: Title, Introduction, Hypotheses, Variables, Methods, Expected Results, Literature Cited, Data Record Sheet. Title The title should be brief but specific. For example: Flower production in full sunlight- and shade-grown sunflowers (Helianthus spp.) You should not include the title as a separate page (save the trees!), but it should head the first page of your proposal. Also include your name, date and pledge on the proposal. Introduction For our purposes, a short paragraph (5 – 10 sentences) that provides a theoretical context for the proposed research will suffice. This will include background information to justify your hypotheses and proposed methods, such as what is currently known about the topic. For example, you might cite a textbook that discusses why sunlight is important for the production of nonphotosynthesizing plant organs such as flowers, or a study showing that sunflowers require more exposure to sunlight than shade-tolerant bluebells (Mertensia spp) for growth and development. 13 Hypotheses Hypotheses are possible explanations for observed phenomena. In your proposal, you will state both a null (HO) and alternate (HA) hypothesis. (Note that in formal lab reports, it is customary to state only HA). HO is the hypothesis of “no difference”, while HA predicts that there will be a difference or association within the data. For example, in our sunflower experiment, we want to investigate whether flower production is enhanced by exposure to sunlight. In this case, the hypotheses might be stated as: HO: Sunflowers grown in full sun produce flowers of equal number as those grown in shade. Another way to say this might be "Light level has no effect on flower production in sunflowers (Helianthus spp.)." HA: Sunflowers grown in full sun produce more flowers than those grown in shade. One cannot “prove” HA, but data can be gathered and analyzed in an attempt to disprove, or test, HO. If our experiment yields data that give us sufficient reason to reject Ho, then we accept HA. For example, suppose we grow 10 potted sunflowers in full sun and 10 potted sunflowers in shade, while keeping all other factors (e.g. sunflower variety, soil moisture or fertility) the same. If, after statistical analysis, we find that the mean number of flowers produced per plant is significantly higher for sunflowers grown in full sun than those grown in shade, then we could reject HO in favor of HA and conclude that sunlight enhances flower production in sunflowers. Conversely, if we found that there was no difference in mean flower production between sun- and shade-grown plants, we would fail to reject HO. This does not mean that we are disproving HA, only that we do not have the data to reject HO. NOTE: While you will always be testing HO statistically, your proposed hypothesis (and the one that you will write about in your final lab report) is really HA. Variables A variable is a characteristic that may differ from one entity to another. In our experiments, we will be testing the effect of an independent variable X on dependent variable Y. Technically speaking, the magnitude of the dependent variable (Y) is assumed to be a function of, or affected by, the independent variable (X). For this reason, the dependent variable is also referred to as the response variable. In the sunflower example, the dependent variable is flower production (mean number of flowers produced per plant) and the independent variable is light level, with the "treatments" within the independent variable being exposure to full sun or shade. Methods 14 In this section, you will describe, in a logical order, exactly what you propose to do. Study site – If you are proposing a field study, you will begin the methods section with a concise description of the study site. It may be appropriate to mention approximately what time of year the study will take place. Experimental Design – Here, you describe as clearly and concisely as possible, the steps necessary to complete your procedure, and to collect and analyze your data. Write this out in paragraph form, not as a list. Your experimental design will outline your variables and treatments (e.g. flower production, sun versus shade), as well as the number of replications per treatment (e.g. 10), often referred to as “n” or “sample size”. As you describe your methods, you will refer indirectly to the materials (equipment, instruments, sample collection materials, etc); do not simply list what you will use. Summarize all necessary equations in numerical format. You do not need to explain details such as, “the data will be compiled in an Excel spreadsheet”, because the reader assumes this. Also you should not include unnecessary details that could vary from study to study, but would not be expected to influence the outcome. Some examples if unnecessary details: • the color of flagging tape used to mark the plants in the field (just say that plants were marked – someone else may use chalk, or a different color tape and achieve the same result) • the length of the measuring tape used to run a transect (here the important detail is simply the length of the transect) • the type of knot used to tie off a strip of dialysis tubing (just say that it was tied off). Statistical Analysis – Prior to data collection, it is important to determine how you will analyze your data statistically. In this class, we will be using either a t-test or linear regression as our primary means of analysis (you will become more familiar with these as the semester progresses). Thus, in your proposed methods, you will write a concise statement about the specific statistical test(s) that will be performed on your data. If using a t-test, you also need to indicate whether you will use a one- or twotailed test. When you conduct your analysis in Excel, it will give you both test values, and it is up to you to specify which one you will use. This will depend upon your alternate hypothesis (HA). Were we to state HA as "Mean flower production will differ between full sun- and shade-grown plants", but not specify which treatment we expect to have greater production, we would use a two-tailed t-test, because we are testing for a difference in mean flower number in either direction (sun > shade or shade > sun). However, in our earlier example, we hypothesized that mean flower production would be 15 greater in full sun plants compared to shade-grown, so we would use a onetailed t-test, which is generally more powerful. Thus, our methods statement with regard to the statistical analysis might be: “Mean flower number per plant will be analyzed using a one-tailed t-test, assuming equal variances, to determine if significant differences in flower number exist between sun- and shade-grown plants”. In some cases we may use linear regression analysis. For example, if we had used a range of light levels (e.g. 100%, 90%, 70%, 50% and 20% exposure to full sun) rather than just two (sun and shade), then we could test whether flower number is related to the amount of light that the plants receive. A regression analysis would test for this relationship. Here, your methods statement with regard to the statistical analysis might be: “Linear regression will be used to determine if flower production in sunflowers is a linear function of light level exposure during growth". (Also refer to your Survival Guide for a primer on statistics) Expected Results – In this section you will provide a concise written statement about each predicted result, along with either a summary table or a summary figure depicting the relative trends you expect from your data. Statements about expected results should be concise but informative and make reference to the appropriate table or figure. For example: "Mean flower production will be significantly higher in sun-grown plants than in shadegrown sunflower plants (t-test: p<0.05)". Tables: In some cases, a data summary table will be more appropriate than a figure, especially if the data are too complex to present graphically. The table should always include a descriptive title above the table, as shown in the example below. The type of summary data that will be inserted (e.g. means ± standard errors or standard deviations) should be indicated. Since you do not have any 'real data' yet, and therefore won't have actual values for your mean, std err or P-value, your summary table will present the trend you expect (e.g. Sun > Shade). Read Chapters 8 & 10 of the Survival Guide for an explanation of means, standard errors and P-values. 16 Table 1. Mean (+ std err) number of flowers produced by sun- and shadegrown sunflower (Helianthus spp) plants. Light Level Flowers/plant Sun Shade Mean + std err > Mean + std err n 10 P-value < 0.05 Note: Tables are easily inserted into the text of a Microsoft Word document using the “Table” menu. This allows you to control the format much better than if you were to cut and paste from Microsoft Excel. Number of flowers/plant Figure – The figure below depicts the same results as the table above. In this example, a figure is a more appropriate way to present the data because the reader can quickly see the expected pattern in flower production (sun > shade). The figure should have a descriptive title caption located below the graph. The caption should concisely describe what the figure represents and include statistics when appropriate. The X- and Y-axes should be labeled with appropriate units. In this figure, the columns have standard error bars, as noted in the figure caption. Sun Shade Fig. 1. Mean (± 1 std err) flower production/plant in sun- and shade-grown sunflower plants (P = ___, n = 10 plants/treatment). In an example where you are testing the relationship between X and Y, using linear regression, a figure would be more informative to the reader than a table. For example, if we had grown the plants under a range of different light levels, a figure would immediately show us the trend in the data (see Fig. 2 below – flower production increased with increasing plant exposure to light). A table with the same data would simply be a list of numbers, and would be more difficult to interpret. The expected results statement might read something like this: There will be a significant positive linear relationship between the level of light exposure plants receive and flower production per plant (R2 = ___, P < 0.05). 17 Number of flowers /plant The accompanying figure might look like this: 0 20 40 60 80 100 Light Level (%) Fig. 2. Flower production per plant in the sunflower (Helianthus spp.) as a function of plant exposure to different light levels (P = ____, R^2 = ___) Literature Cited – Sources cited (by author, year) in the Introduction section of your proposal will appear in the Literature Cited section as full bibliographic citations (refer to Chapter 7 in the Survival Guide for proper formatting). Data Record Sheet – Designing a data record sheet before you conduct your research helps you better understand your experimental design and prepares you for more efficient data collection. This is a table where you will record your raw data during the study and is different from a summary table of the results. Your data record sheet should reflect the set up of the spreadsheet that you will use to enter and analyze the data in Microsoft Excel. Example of an abbreviated data record sheet (you should include an entire data record sheet in your proposal) Plant number 1 2 3 ... 10 1 2 3 ... 10 Light treatment sun sun sun sun sun shade shade shade shade shade Number flowers/plant 18 Proposal Format: 132 Perspective • All sections in your proposal, outlined below, should be identified with corresponding headings. • Use FUTURE TENSE consistently throughout the text when referring to your proposed research, as you are writing about activities that you will conduct in the future. Note that this is in contrast to your lab report, in which you will use the PAST tense, because your research will be complete when you write the formal report. • If you are working in a group, use first person plural (we), otherwise use first person singular (I). E.g. We/I will measure the rate of enzyme action in solutions with pH measurements of six and eight. The passive voice may also be used. E.g. The rate of enzyme action will be measured in solutions with pH measurements of six and eight. • All text within your proposal, including the methods, should be written in concise, but complete, sentences and organized into paragraphs. • When providing the scientific name of a bacterium, plant or animal for the first time, remember to use upper case (capitalize) the first letter of the genus (the species remains in lower case), and either underline or italicize both the genus and species. After the first citation, you may simply refer to the common name, or the abbreviated scientific name. For example, “We propose to examine the enzyme tyrosinase isolated from the potato, Solanum tuberosum)”. Thereafter you can just refer to the organism as the potato or as S. tuberosum. • Number each page, and staple, or paper clip the pages together, before you hand your proposal. Reuse and recycle paper where possible. Printing your work on the clean side of used paper is perfectly acceptable, so long as your work is readable. • A sample proposal available online at the lab web site demonstrates the desired format, writing style, and approximate text length. • The Student Survival Guide (available as a download from the course lab web site) contains additional information on data collection, proposal and report writing, and on using Microsoft Excel for data analysis. PROPOSAL CONTENT The proposal does not have to be long, but it should be complete, and include all of the following components: Title, Introduction, Hypotheses, Variables, Methods, Expected Results, Literature Cited, Data Record Sheet. 19 Title The title should be brief but specific. For example: The effects of lowering pH on the rate of activity of the enzyme, tyrosinase. You should not include the title as a separate page (save the trees!), but it should head the first page of your proposal. Introduction For our purposes, a short paragraph (5 – 10 sentences) that provides a theoretical context for the proposed research will suffice. This will include background information to justify your hypotheses and proposed methods, such as what is currently known about the topic. For example, you might cite a textbook that discusses why level of pH is important for the enzyme reaction rate, in general, or a study showing that the reaction rate of tyrosine is affected by pH in another organism, such as portabello mushrooms. Hypotheses Hypotheses are possible explanations for observed phenomena. In your proposal, you will state both a null (HO) and alternate (HA) hypothesis. (Note that in formal lab reports, it is customary to state only HA). HO is the hypothesis of “no difference”, while HA predicts that there will be a difference or association within the data. For example, in our enzyme reaction experiment, we want to investigate whether enzyme action is affected by a decrease in pH. In this case, the hypotheses might be stated as: HO: The enzyme tyrosinase, when buffered in solutions of pH 6 and pH 8, reacts with the substrate catechol to produce a colored product at similar rates. HA: The enzyme tyrosinase reacts more slowly with the substrate catechol to produce a colored product when buffered at pH 6 than it does at pH8. One cannot “prove” HA, but data can be gathered and analyzed in an attempt to disprove, or test, HO. If our experiment yields data that give us sufficient reason to reject Ho, then we accept HA. For example, suppose we combine 4 ml of tyrosine and 4ml of catechol buffered in a sodium phosphate solution with a pH of 6 and compare the rate of color formation with 4 ml of tyrosine and 4 ml of catechol buffered in a sodium phosphate solution with a pH of 8. If, after analysis of our data, we find that the rate of reaction is significantly higher for enzyme buffered at pH 8 than for enzyme buffered at pH 6, then we could reject HO in favor of HA and conclude that a pH of 8 is more optimum for action of the enzyme tyrosinase than a pH of 6. Conversely, if we found that there was no difference in reaction rates of enzymes in buffers of pH 6 and pH 8, we would fail to reject HO. This does not mean that we are disproving HA, only that we do not have the data to reject HO. 20 NOTE: While you will always be testing HO statistically, your proposed hypothesis (and the one that you will write about in your final lab report) is really HA. Variables A variable is a characteristic that may differ from one entity to another. In our experiments, we will be testing the effect of an independent variable X on dependent variable Y. Technically speaking, the magnitude of the dependent variable (Y) is often assumed to be a function of, or affected by, the independent variable (X). For this reason, the dependent variable is also referred to as the response variable. Independent variable treatments are the various levels or values of independent variable X that you plan to test. They should be specified in your proposal along with the independent variable. In the enzyme example, the dependent variable is enzyme reaction rate and the independent variable is the pH level of the buffer to which the enzyme is added, with the treatments within the independent variable being the various pH levels you plan to test (eg. pH 6.0, 7.0, 8.0, 9.0 and 10.0). Methods In this section, you will describe, in a logical order, exactly what you propose to do. Experimental Design – Here, you describe as clearly and concisely as possible, the steps necessary to complete your procedure, and to collect and analyze your data. Write this out in paragraph form, not as a list. Your experimental design will outline your variables and treatments (e.g. enzyme reaction rate, pH 6 versus pH 8), as well as the number of replications per treatment (e.g. 10), often referred to as “n” or “sample size”. As you describe your methods, you will refer indirectly to the materials (equipment, instruments, sample collection materials, etc); do not simply list what you will use. Summarize all necessary equations in numerical format. You do not need to explain details such as, “the data will be compiled in an Excel spreadsheet”, because this is assumed by the reader. Also you should not include unnecessary details that could vary from study to study, but would not be expected to influence the outcome. Some examples of unnecessary details: • • • • the size of the test tube into which you mixed your enzyme and buffer solution the fact that you had to push the blue button to turn on the spectrophotometer the type of knot used to tie off a strip of dialysis tubing (just say that it was tied off) the number of each tube. The important detail here is what is in each tube (e.g. the solution, and/or its concentration) or its treatment (e.g. boiled, frozen, untreated) Statistical Analysis – Occasionally, you will be required to analyze your data statistically, but for the most part in Biology 132, your comparisons will be qualitative rather than quantitative. If you are required to conduct statistical analysis, then prior to data collection, it is important to determine how you will analyze your data statistically. In this class, we will most likely be using 21 either a t-test or linear regression as our primary means of analysis (you will become more familiar with these as the semester progresses, if necessary). Thus, in your proposed methods, you will write a concise statement about the specific way in which you will analyze your data. For example, is it a qualitative comparison of reactions rates, or mean heart rates, or does your analysis involve a statistical comparison of reaction rates or mean heart rates. If it is a statistical test analysis, what test are you using. In this latter statement, you should include your predetermined alpha (α), or “significance” level, which indicates the level of risk you are willing to accept when declaring statistical differences among treatments. Generally, this value is ranges from 0.001 to 0.05, or may be as high as 0.10, depending upon the type of experiment (and the stakes involved). An α = 0.05 means that there is a 5% chance of finding a significant difference among treatments by random chance, when in fact, this difference does not exist. In other words, you have a 5% chance of rejecting HO when HO is really true. Your lab instructor will let you know if a statistical test is needed, and what the appropriate α level is. Also refer to your Survival Guide, Chapter 9 for a primer on statistics. Expected Results – In this section you will provide a concise written statement about each predicted result, along with either a summary table or a summary figure depicting the relative trends you expect from your data. Statement: The expected results statement should be concise, but complete, and include an indication of the general trend you expect to find, based on your alternate hypothesis. For example: "The rate of enzyme action will be higher in a solution of pH 8 than in a solution of pH 6 (Table 1)". Tables and Figures: In addition to the results statement (to which you should make reference in your expected results statement), you will need to include a summary of your results, either in the form of a table or a figure. Tables are more appropriate if the data are too complex to present graphically. The table should always include a descriptive title at the top of the table, as shown in the example below. Means ± standard errors, or standard deviations, when appropriate, are included, and identified as such in the table title. Since you do not have any 'real data' yet, and therefore won't have actual values for your mean, std err or P-value, your summary table will give present the generalized format of how your actual data will appear in your formal lab report. Read Chapters 7 & 9 of the Survival Guide for an explanation of means, standard errors and P-values. 22 Table 1. Mean (+ std err) reaction rate of the enzyme tyrosinase extracted from potato (Solanum tuberosum) with catechol substrate when buffered at varying pH levels. Reaction rate pH 6 pH 8 Mean + std err > Mean + std err n P-value 10 < 0.05 Note: Tables are easily inserted into the text of a Microsoft Word document using the “Table” menu. This allows you to control the format much better than if you were to cut and paste from Microsoft Excel. Reaction rate Absorbance (min) Figure – The figure below depicts the same results as the table above. In this example, a figure is a more appropriate way to present the data because the reader can quickly see the magnitude of the different in reaction rates at the two pH levels. The figure should have a descriptive title located at the bottom of the graph. The title should include statistics, when appropriate. The Xand Y-axes should be labeled with appropriate units. In this figure, the columns have standard error bars. 8 6 buffered pH Fig. 1. Mean (± 1 std err) reaction rate of tyrosinase with catechol substrate (P = ___, n = 10). Tyrosinase enzyme was isolated from potato ( Solanum tuberosum ) immediately prior to use. In an example where you are testing the relationship between X and Y using linear regression, a figure is definitely more informative to the reader than a table. For example, if we had examined reaction rate over a range of different pH levels, a figure would immediately show us the trend in the data (see Fig. 2 below – enzyme activity increases with increasing pH). A table with the same data would simply be a list of numbers, and would be more difficult to interpret. The expected results statement might read something like this: There will be a significant positive linear relationship between the activity of the enzyme tyrosinase and pH of the buffered substrate (Figure 2). The accompanying figure might look like this: Absorbance (min) Reaction rate (¶ 23 0 2 4 6 8 10 12 Buffered pH Fig. 2. Reaction rate of the enzyme tyrosinase with catechol substrate a function of buffer pH (r2 = ___, P < 0.05). Tyrosinase was isolated fpotato (Solanum tuberosum) immediately prior to use. Literature Cited – Sources cited (by author, year) in the Introduction section of your proposal will appear in the Literature Cited section as full bibliographic citations (refer to Chapter 7 of the Survival Guide for formatting). Data Record Sheet – Designing a data record sheet before you conduct your research helps you better understand your experimental design and prepares you for more efficient data collection. It also helps prepare for analysis after you have collected the data: Your data record sheet should reflect the set up of the spreadsheet that you will use to enter and analyze the data. Example of an abbreviated data record sheet (you should include an entire data record sheet in your proposal) Data record sheet for 1 potato sample: repeat table for each of 10 samples. pH 6 pH 8 Minute Elapsed Abs.** ∆ Abs.** Minute Elapsed Abs. Time Time (min:sec) (min:sec) 0 1 2 3 10 0 30 1:00 1:30 2:00 2:30 15 45 1:15 1:45 2:15 2:45 3:00 ... 10:00 3:15 ... 10:00 Reaction rate (/min) = ______________ ∆ Abs. Reaction rate (/min) = ______________ 24 Sample Research Proposal Name: Pledge: Title Flower production in full sun- and shade-grown sunflowers (Helianthus spp.) Introduction Plants are photoautotrophs that use light energy to drive the process of photosynthesis, although the amount of light necessary for normal growth and development vary among species. Shade intolerant plant species have higher light compensation points (LCPs) than shade tolerant plants, which means that they need a greater amount of photosynthetically active radiation (PAR) for simple maintenance respiration and growth (Kozlowski et al. 1991). Beaubaire (1997) found that wild ginger (Asarum caudatum), which normally grows in dense carpets in the understory of redwood forests, suffered photoinhibition and high mortality when grown in full sun, while light-demanding cardinal flowers (Lobelia cardinalis) would not grow in less than 70 percent full sun. In general, sexual reproduction requires a large energy investment by the plant that is often dependent upon stored reserves because organs such as flowers and fruits do not photosynthesize (Pearcy et al. 1989). Common sunflowers (Helianthus spp.) are a plant favored by many flower gardeners. Because most residential gardens in Sewanee, Tennessee, are shaded by oak trees, we propose to examine whether a lack of sunlight would greatly reduce flower production by common sunflowers. Because flower production requires a large energy investment by any plant, we will also examine whether mean flower mass per plant decreases as the number of flowers produced on full sunlight-grown plants increases. Hypotheses 1. HO: Sunflowers grown in full sunlight produce the same number of flowers per plant as those grown in shade. HA: Sunflowers grown in full sunlight produce more flowers per plant than those grown in shade. 2. HO: Flower mass of full sun sunflower plants is not a function of the number of flowers produced per plant. HA: Flower mass of full sun sunflower plants decreases as the number of flowers per plant increases. Variables Hypothesis 1: Dependent variable = flower number (# of flowers produced per plant) Independent variable = sunlight exposure Independent variable treatments = exposure to full sunlight vs. 85% shade Hypothesis 2: Dependent variable = mass (g) of flowers produced on full sun-grown plants 25 Independent variable = number of flowers produced per full sun-grown plants Methods Experimental Design. To compare flower production between sun- and shade-grown sunflower plants, we will expose ten 4-week old sunflower plants to 8 hours of full sunlight in a greenhouse for a total of 30 days. An equal number of the same variety of sunflower plants will be grown in the same greenhouse under a mesh cloth which permits only 15% PAR emission. We chose 85% shade for this treatment because it approximates the conditions of typical tree-covered gardens of Sewanee residences. Prior to treatment, the plants will be germinated and grown in the same 40% loam potting soil mix and will be watered daily with 500 ml of 0.5 M Hogan’s fertilizer solution to ensure that nutrient deficiencies do not inhibit flowering in either treatment. After 30 days, we will count the number of flowers on each plant. The flowers will then be excised at the base of the flower head, placed into separate labeled paper bags, and dried at 60 degrees C° for 48 hours. The dried flowers from full sun-grown plants will be weighed to quantify mean flower dry mass per sunflower plant. Statistical Analyses. Mean flower number per plant will be analyzed using a one-tailed t-test, assuming equal variances, to determine if sun-grown plants produce significantly more flowers than shade-grown plants. Mean flower weight per plant from full sun-grown plants will be used in linear regression analysis to determine if flower mass is a function of the number of flowers on a plant. Expected Results Effect of light level on flower production – The mean number of flowers produced per plant will be significantly higher in sun-grown than in shade-grown sunflowers (P <0.05, Fig. 1). 14 12 10 8 6 4 2 0 Full sun Shade Fig 1. Mean flower production (± std err) in full sun- and shade- grown sunflower (Helianthus spp) plants (n=10, p = 0.014) Effect of flower production on flower size – Mean flower mass for sun-grown plants will decrease with increasing number of flowers produced per plant (P < 0.05 , R2 = #.## , Fig. 2). 26 0 Number of flowers per plant 0 Fig. 2. Mean flower mass (g) of sunflower plants (Helianthus spp.) grown in full sun as a function of the number of flowers produced per plant (P = ___, R^2 =___). Literature Cited Beaubaire, J. 1997. Sun requirements for wildflower gardens. Accessed Oct. 13, 1998. http:/www.bbg.org/topics/wildflowers/html. Kozlowski, T. T., P.J. Kramer, and S.G. Pallardy. 1991. The Physiological Ecology of Woody Plants. Academic Press Inc., San Diego, CA, USA. Pearcy, R.W., J. Ehleringer, H.A. Mooney, and P.W. Rundel. 1989. Plant Physiological Ecology. Field Methods and Instrumentation. Chapman and Hall, London, England. Data Record Sheet NOTE: there is no fixed design for a data sheet – use your creativity, but strive for clarity, ease-of-use during data collection, and ease-of-transfer of data into the computer later. Your data sheet will be empty until you collect your data but in the example below some sample numbers have been inserted to illustrate how the sheet will be used. Plant No. 1 2 3 4 5 6 7 8 9 10 No. flowers Full sun Shade 7 12 2 1 Mass per full sun-grown flower (g) 25, 30, 22, 18, 15, 22, 25 17, 12, 15, 16, 8, 20, 13, 15, 20, 17, 22, 9 Mean flower mass (g) 27 CHAPTER 5: A GUIDE TO WRITING SCIENTIFIC PAPERS 1 This guide is intended to supply the student with a model for writing a paper on scientific research. Consider this guide to be an acceptable starting point. It will work well for any class taken in this Department of Biology. Goals The writing of scientific paper is based on several simple concepts. The main purpose of a scientific paper is to tell the reader why you did the experiment, what you did, how you did it, the results, and the significance of those results. The style emphasizes conciseness and clarity of language, so words must be chosen to say exactly what you mean them to say, with no tolerance for vagueness or "you know what I mean." The organization of a paper is logical, with rules for what goes into each section. Finally, factual material (results) and the interpretation of factual material (discussion) are clearly separated into different sections and never allowed to become confused. Remember these goals as you write a paper, and judge your own work by them before you complete it. In addition, a paper should be written in PAST TENSE as it is something that you have already done. Sections of a Paper Though individual papers will vary in some details, the basic arrangement of papers in this course is usually the following: Title Abstract Body of the Paper: Introduction Materials and Methods Results Discussion Literature Cited Tables, Figures and Legends Each section, other than the title page, should bear the heading for that section, e.g. “Materials and Methods." Papers should be paginated with the title page as page one and all subsequent pages numbered accordingly. Title ◊ Title should appear at the top and be brief but should inform the reader of the subject (but usually not the results) of the work being reported. Be specific. "Franklin County Flora" is too broad for a project that is really "Differences in Flora at Different Elevations in Franklin County, Tennessee." ◊ The author's full name and the full names of all partners with whom data were shared (if any). ◊ The date. 1 Adapted from a version written by Dr. Timothy Keith-Lucas and used with his permission. 28 ◊ The pledge and signature of the author only. Abstract The abstract should be on the same page just after the title. The abstract is a brief (approximately 150-250 words) summary of the entire paper. The simplest way of writing an acceptable abstract is to summarize in turn each of the four major sections of the report (Introduction, Methods, Results, and Discussion) in, at most, two sentences each. Don't forget to include the results here. Introduction A typical (it varies) Introduction has three parts. First, in a brief paragraph, give general information relating to your topic. This should also include a general question or issue of interest. Note that this may be a general question about the way the world works, rather than a narrow question applicable only to one locality. You may be attempting to answer this question in a very narrow set of circumstances in one place, but your interest as a scientist is in broader questions about the world. It is most important, not just to the paper but to the success of the project in general, that this question or goal be clearly stated. Having introduced a topic, now describe what is known about the topic prior to the beginning of your project. Typically, this part is a literature review, describing what others have found when they have studied similar issues in other locations. This section might include your logic for suspecting a particular result. Be sure to cite properly work which has already been published (see the Literature Cited section of this guide). Do not go into the details of Methods, report results or reach conclusions in the Introduction. Finally, write a short paragraph telling the reader what your specific hypothesis is, and how you tested the hypothesis you have posed and reviewed above. "By analyzing the flora at five elevations in Franklin County, Tennessee, we tested the hypothesis that flora composition varied with slight changes in elevations" could be the lead sentence in this section. Be brief, and leave the details for the Methods section. Note the logic in the paper thus far. Introduce the reader to the topic, describe what is already known, and finally, state your hypothesis and how you intend to test it. Now the reader is ready to read the details of your work and will understand the intent behind your use of various methods. Materials and Methods In this section you need to describe your work in sufficient detail to allow the reader to replicate the study. That means that any detail that could influence your results needs to be included. An example would be the size of the sample taken or the season in which the study was performed, what solutions you used and what their concentrations were. The reader may need to read instruction manuals for equipment used, but they should know what equipment to use, volumes, weights, doses, settings, numbers of subjects and treatments, etc. Details that would not reasonably be expected to influence the results should be excluded. This requires judgement. You must ask yourself whether a detail could influence the results, and you must describe an experiment clearly to someone who was not present when you were. 29 Describe the steps taken to complete the study. Avoid giving instructions ("Dip the pH meter in the sample.") when procedurally what happened was that the pH was measured. Again, include everything necessary to replicate the work, and nothing more. This is NOT to be written giving instructions as if it is a laboratory manual. Each step of the methodology should have its own paragraph and each paragraph should have a topic sentence, other sentences that are on that topic and give more details, and then a final sentence. A methods section that starts with “These are the methods I will use” and then goes on in one long paragraph is not acceptable. Methods should include any formulas that you used for calculations. Include not only the formula with the variables [with all variables defined, e.g. M is the mass (g)] but also one sample of the formula with all the numbers substituted for the variables. Do this for all the formulas you use (except statistical formulas). Any statistics you use should be stated in the methods. For statistics, the formulas are generally well known, so you do not need to give statistical formulas, just state that you found the mean + S.E. (standard error or standard deviation – S.D. or whatever) and any test that you used to determine statistical differences between values (t-test, ANOVA, etc.). Again, note the logic. Now you have described a specific experiment with which you hope to answer the question posed and reviewed in the Introduction. Results In the Results section describe what you got when you carried out the procedure just described in the Materials and Methods section. Report the results factually (no interpretation). Be quantitative (use the numbers), and leave your conclusions ("...because temperatures are lower at high elevations") for the Discussion. The results will not be merely a compilation of tables and graphs. Although most of your results will appear in tables and figures (for example, graphs or drawings), a person reading the text of your Results section should understand the results of your study without having to study your figures and tables. Figures should support the text and supply details, but what happened should be clear from the text itself. Never, ever, start a Results section with "The results are summarized in Figures 1 through 4." When used, figures and tables should clearly make your point, usually by comparing the results found under two or more conditions, and should be clearly labeled and cited in the text where appropriate. Avoid the temptation to pile in irrelevant figures. Refer to figures and tables in the text of your Results by their numbers. You do not have to write “See Figure 1,” just say something like “The osmotic concentration of the bodily fluids was higher in x than in y (Figure 1).” Figures and tables should be numbered separately, a series of numbers for figures and a series of numbers for tables. In addition, they should be numbered in the order in which they are referred to in the text. Never refer to Table 2 before you refer to Table 1. Renumber your tables if you need to!! Never have a table or a figure to which you do not refer your reader!! You must make reference to all tables and figures. 30 Each table and each graph must be formatted properly. Tables should have a number and title above it (Table 1: Absorbance for pigment A at each wavelength tested.). Units should be found in Column titles (Wavelength (nm)). Any additional information such as abbreviations should be in a caption found below the table. Graphs or figures should also have a number and title, although these are often found below them as a caption (Figure 1. Absorption spectrum for pigment A.) Additional information, such as what each type of symbol or color of line means and any abbreviations should be given in a legend. In addition, each axis must have a label, and most parameters will have units. Tables and figures should be found on separate sheets at the end of the paper. The table number and title with all needed information (such as abbreviations, etc.) should be on the same page as the table. The figure number should be on the same page as the figure. A list of figure legends can all be on one separate sheet at the end of the paper, or each figure legend can be on the same page as the figure. This is the way scientific journals require manuscripts to be formatted when submitted for publication. Discussion You have stated a hypothesis, described a specific experiment to address it, and reported the results of that experiment. Now it is time to discuss whether or not the data support the hypothesis. In the first paragraph you should start with a restatement of your hypothesis, why it was posed and whether or not your data support or do not support the hypothesis. It is best not to keep your reader guessing about whether or not the hypothesis was supported. In subsequent paragraphs, you should argue for your conclusion(s) with all the logic and data (from the results) that you can command. NEVER say that you have proven ANYTHING in your paper. You have not. You have merely gathered evidence that either supports you hypothesis (fails to reject) or fails to support (rejects) your hypothesis. You must also deal with alternative explanations for the results (such as having observed at different times of day in the two sites) and with weaknesses in your design, as in "The study would have been improved by a larger sample." If your conclusion is that your hypothesis was wrong, say so and why. In addition, data are never wrong (as long as there were proper controls and the methods were followed properly). They are just your data. Do not do a lot of data-bashing in your discussion. There is not a requirement that research turn out the way you expected it to. It is far more scientific to report your data accurately than to obtain conclusive results. It is OK to pose further hypotheses to be tested in the future. Literature Cited You are required to learn how to cite references, both in the text and at the end of the paper. The style presented here is the same as that used in the journal Ecology. Notice that it is different from styles used in English and other humanities courses. Refer to Chapter 7: Literature Cited for instructions and examples of correct citations to be used in all assignments. 31 Note that you have come full circle. You started with a question and ended with the answer to it or with a hypothesis and ended either supporting or failing to support it. Thus is the logic of report writing. Tables and Figures Tables and figures are elements of your work that allow you to summarize (tables) or graphically represent (figures) your data in a way that will give your reader a ‘bottom line’ understanding of your work. Each has it’s own major parts with some important guidelines to remember. Tables within a paper should be numbered (Table 1, Table 2 etc.) in the order that they appear in the paper. Number and title are located directly above the table. Titles should be unique for each table, and should tell the reader about the data. (Table 1 Index of similarity for diets of 3 sympatric salamander species.) Located above each column is a column heading, identifying the data in the column; usually just a word or short phrase. These are commonly the dependent variables. Row headings are found in the first column of the table. This column is also called the “stub”. These headings are often the independent variables and are also just a short phrase. The body of the table, or the field, contains the data. Footnotes, denoted with a symbol and located directly beneath the table, should be used to clarify notations or other ambiguities in the table. (* Comparisons were made using the five most abundant prey items for each species.) Figures are also numbered in the order in which they appear in a paper (Figure 1, Figure 2 etc.) and have a title as in tables. The caption consists of the Figure # and the title together, and is also a phrase or sentence fragment describing what the data show. (Figure 1. Five most abundant prey items for 3 sympatric species of salamander.) The caption is located below a figure. You’ll use figures mostly to illustrate trends or proportions, but they also include photographs, drawings or other illustrations. Axes as well as legends should be clearly labeled including units for axes and symbols used in legends. Tables and figures should be indirectly referenced in the text in which they are discussed using parentheses and the figure or table number (Table 2) or (Fig. or Figure 2). Formatting In scientific papers as in proposals, certain things need to be formatted very specifically. Please refer to Chapter 5 Research Proposal Guidelines for further format guidelines and examples. 33 CHAPTER 6: LITERATURE CITED You are required to learn how to cite references, both in the text and at the end of the paper, according to the format used in the peer reviewed journal Ecology, as adopted by this department. Notice that it is different from styles used in English and other humanities courses. Citations Within Text There are several ways to cite the sources of ideas within the text of the report. Either they are numbered and the number of the reference is found in the text (e.g. as in Science and Proceedings of the National Academy of Science), or they are cited by the name-and-year system (most biology journals). You will use the name-and-year system for Biology 131 and 132. Following are some examples of how to cite references within text. 1. When there is one author: "Jones (1983) showed that . . ." or "Previous work (Jones 1983) shows . . ." 2. When there are two authors, use the last names of both authors when citing, as in "Jones and Doe (1983) reported . . ." or "as previously reported (Jones and Doe 1983) . . ." 3. When there are more than two authors, use only the last name of the first author, followed by et al., as in "Jones et al. (1983) report that . . ." or "Some investigators (Jones et al. 1983) have reported . . ." NOTE the format of et al.: it is italicized and there is a period after al. The expression et al. is Latin for "and others." 4. When there are two or more references showing similar results, list the references chronologically, separated by a semicolon: "This has been previously reported (Jones 1983; Smith and Doe 1985) . . ." The format of citations must be followed precisely; down to the commas and the use of italics. Literature Cited Page At the end of the paper you will have a Literature Cited section. The format of your citations must conform to the following formats. Pay close attention to what is or is not capitalized or italicized, where periods, commas, colons and semicolons appear, and the order in which the information (such as authors and their initials, etc.) appears in the citation. With regard to Authors’ names – use initials only, do not give full first and/or middle names. Also, the authors must be in the proper order. The first author listed on a paper or book must be the first one you list within the citation. In your Literature Cited section, alphabetize entries according to the last name of the first author of each work cited. If you have multiple sources from the same author(s), arrange by 34 year (earlier first); if you have multiple sources from the same author(s), in the same year, add a lower case letter to identify each when citing in the text and when making the bibliographical entry (Jones 1983a, Jones l983b). In addition to the formatting discussed above, Latin names, including species names, are underlined or italicized. Note that the last names of all authors of a paper are included in the Literature Cited section, even though the names of only one or at most two authors are cited in the text of the report (e.g.: “Berner et al. 1999” would be found in the text, but “Berner, N.J., D.A. Grahn, and H.C. Heller. 1999. 8-OH-DPAT-sensitive neurons in the nucleus raphe magnus modulate thermoregulatory output in rats. Brain Research 831:155-164.” would be found in the Literature Cited section.) Standard Journal Article Single author Generic format: Author #1, A.A. Year. Title of article. Title of Journal Volume:pages. Example: Haskell, D.G. 1995. Re-evaluation of the effects of forest fragmentation on rates of bird-nest predation. Conservation Biology 9:1316-1318. Two or more authors (separate last and next to last author names by 'and') Generic format: Author #1, A.A., B.B. Author #2, and C.C. Author #3. Year. Title of article. Title of Journal Volume:pages. Example: Berner, N.J., D.A. Grahn, and H.C. Heller. 1999. 8-OH-DPAT-sensitive neurons in the nucleus raphne magnus modulate thermoregulatory output in rats. Brain Research 831:155164. Books Note: the title of the book is neither underlined nor italicized. Personal Author(s) (e.g. the entire book is written by the author(s)) Generic format: Author #1, Author #2 and Author #3. Year. Title of book. Edition (if applicable). Publisher, City, Country. Example: Eason, G., C.W. Coles, and G.Gettingby. 1980. Mathematics and statistics for the biosciences. 5th ed. Ellis Horwod Limited, West Sussex, England. Edited book (e.g. a book in which different authors contributed individual chapters) Generic format for citing an individual chapter: Chapter Author #1 and Chapter Author #2. Year. Title of chapter. Pages XX-YY in Editor(s). 35 Title of book. Edition (if applicable). Publisher, City, Country. Example: Hammerschmidt, R and R.L. Nicholson. 1999. A survey of plant defense responses to pathogens. Pages 55-71 in Agrawal, A.A., S. Tuzun and E. Bent, editors. Induced plant defenses against pathogens and herbivores: biochemistry, ecology and agriculture. APS Press, St. Paul, USA. Example for citing the entire book (rather than an individual chapter): Wood, R.K.S., editor. 1982. Active defense mechanisms in plants. Plenum Press, New York, USA. Items From The World Wide Web 1 You should be cautious when looking for sources on the internet. It is important that articles used are from reputable, peer reviewed web sites. Remember, any Billy Bob can put anything they want to, information or misinformation, on the world wide web. Note that articles that are accessed online from journals that are also available in print version (e.g. Ecology, Conservation Biology, Molecular Biology of the Cell) should be cited according to the printed journal format given above, and not as a web document. Only periodicals that are available only as an electronic source should be cited as web documents. When citing web sites, include the author(s), year (if available), title of web page, retrieval date of the information and the Universal Resource Locater (web address) or URL. Generic format: Author, A.A. Year. Title of work. Retrieved month day, year. URL. Note: If the web site does not have an obvious author, cite the author as Anonymous Example: Anonymous. Year. Title of work. Retrieved month day, year. URL. Brown, J. 1996. Bugs in the News. Retrieved October 21, 1996. http://falcon.cc.ukans.edu/~jbrown/bugs/html. 1 Pechenik, J.A. A Short Guide to Writing About Biology. 3rd Edition. Longman Press, N.Y. 1997. 36 CHAPTER 7: AN INTRODUCTION TO GRAPHS AND DATA SUMMARIES This tutorial will introduce you to some of the tools that biologists use when we present and summarize their data. Part 1: What items are considered data? Before you jump into drawing graphs and calculating averages, it is important that you understand the nature of your data. Here are the three main categories: Continuous data These are numbers that can take on any value (i.e. fractions are allowed). For example: • • • • human height measured with a ruler temperature measured with a thermometer diameter of hickory nuts measured with calipers proportion of time a squirrel spends feeding Discrete data These are all non-continuous data. There are two main classes: Categorical Data: These occur in non-overlapping, distinct categories or classes. These categories are not usually numerical. For example: • • • color of bird feathers classified into red, blue or brown sex: male or female a bird nest classified as either having been preyed upon, or having successfully reproduced young birds. Counts: This type of data is numerical, but not continuous • the number of acorns found in a square meter of forest. Here we cannot have “half acorns”, so the data are not continuous. • the number of birds seen on a census Part 2: Show me your data! Descriptive statistics Descriptive statistics should convey information about data in an easy-to-understand and digestible way. We encounter descriptive statistics everyday: Grades and GPA’s are supposed to summarize the results of countless tests and papers, stock market indices (e.g., the Dow Jones average) summarize the performance of some aspect of the stock market, “last frost” dates are used by farmers and gardeners to plan their planting, newspapers publish “crime statistics” which may summarize criminal activity in our communities. All these summaries are descriptive statistics. Later in the course you will study inferential statistics or "statistical tests" which are designed to test for differences between groups, or look for correlations between variables. There are many methods of summarizing data, and our choice of method will affect the message that we convey with our data. Both producers and consumers of descriptive statistics should know how the choice of descriptive statistic affects this “message”. 37 Producers of information should be aware so that they can choose the most appropriate (or deceptive, if you are in the spin-doctoring trade!) method. Consumers of information should be aware so that they can evaluate the information that is given to them. For example, if a College claims that its “average class size is 12”, the question that should jump to the front of your mind is: what kind of average? The nitty-gritty: when we are summarizing data we might want to convey information about the following: ♦ the “average” ♦ how much variation is present in the data ♦ trends in the data ♦ relative proportions of categories in the data The “average” of continuous numbers and counts can be summarized as follows: Mean: Add up all the numbers in your sample and divide by the sample size. The mean gives a measure of the “center of gravity” of data. You can use this with continuous or count data. Median: Line up the numbers from biggest to smallest (i.e. put them in rank). The number in the middle is the median. The median is also called the 50th percentile. You can use this with continuous or count data. Mode: This is the most common number. For categorical numbers you can report the mode (the category with the largest number of entries) but not the median or the mean. Variation can be measured in the following ways: Range: This is the difference between the two extreme values in a sample Standard deviation (s.d.): This is a more involved measure of the variation in a sample. To get the s.d. we calculate the difference between the value of each data point and the mean of all the data points. Although it is not important for our purposes to know the exact formula, the s.d. is the square root of these values divided by the sample size. The important thing to note is that the s.d. is larger in more variable datasets than in more uniform datasets. Example: Here are some continuous data arranged in rank: 0, 0, 0, 0, 1, 1, 1, 1.3, 1.4, 1.5, 1.9, 2, 2.4, 2.4, 2.6, 3, 3.5, 3.5, 4.3, 5, 6.5, 8, 9, 9.7, 12 The sample size is 25 The mean of this data is 3.36 The median is 2.4 The mode is 0 The standard deviation is 3.37 The range is 12 Look at that! The mean is higher than the median. This happened because our data are skewed (skewed = not arranged symmetrically around the mean). Now, if you were an employer hoping to attract a new employee and these data represented the salaries of employees in your company in units of $10,000, you might use the mean and say “The 38 average salary in my company is $33,200”. On the other hand, if the data represented student debt after 1 year in college in $1000 units, a college recruiter might use the median and say “The average student debt after one year at our College is only $2,400”. Neither person lied, both reported real averages. So, which is the “right” average to use? The answer to this question depends what you want your summary statistics to represent. The median will always have half the data points above it and half the data points below it. The mean will show the “center of gravity”. In general, if the frequency distribution of your data is bell-shaped, the mean and the median will be about the same. If you have one or two very extreme values in your data (e.g., in the example above if we had a value of 120), the mean will be strongly affected by this value, whereas the median will not. To plot the mean and standard deviation on a graph use a “dot” for the mean, and two lines for the standard deviation. Each line is exactly one standard deviation long as the following graph shows: 8.00 Salary in Thousands 7.00 6.00 5.00 4.00 3.00 2.00 1.00 0.00 Figure 1. Summary of our data (showing mean and one standard deviation). This graph only has one data point and therefore does not convey much information. Graphs are most useful when you want to summarize large amounts of data in an easy to read format. Before you go on to study some different types of graph, please review the following guidelines which apply to all graphs: General guidelines for all graphs i. ii. iii. Label each axis, include the units of all measurements. Give the graph a title. The title should do more than repeat the axis labels. Scientific journals do not publish 3-D graphs unless there really are three axes. 3dimensional graphs may look fancy, but they are hard to read. 39 Overview of three types of graph: In this section you will learn about scatter plots, line graphs, and bar charts. Each type of graph is useful in a different situation. Here is a quick summary of when to use each type of graph: Table 2. Basic graph types and when to use them. Graph type Scatter plot Use this graph… For continuous or count data: shows relationship between two sets of numbers For continuous or count data when we want to look at the details of "ups and downs" in the data. When data are arranged in categories and you wish to present a summary for each category Line graph Bar chart Scatter plot: Usually used when we want to show the relationship between two groups of numbers that are continuous or count data. For example: Volume (mm) 35 30 25 20 15 10 5 0 0 5 10 15 Age (hours) 20 25 Figure 2. Volume of milk consumed by day-old piglets plotted against piglet age. Sometimes we may want to draw a “best” line through the points. One such method is the “best straight line” method. Be careful - only use “best straight lines” when you have good reason to expect a linear relationship. A common mistake is drawing straight lines through data that are not linear. Here are the piglet data with a best fit line drawn through them. There are statistical methods that allow precise drawing of best fit lines -- the most common is the called "least squares" method. We will discuss this method in more detail in the section on inferential statistics. 40 35 30 25 Volume (ml) 20 15 10 5 0 0 5 10 15 20 25 Age (hours) Figure 3. Volume of milk consumed by day-old piglets plotted against piglet age (with best fit line). Line graph: This is essentially a scatter plot with the “dots” connected. This graph is usually used when we want to show how one variable (on the y-axis) varies with changes in another (on the x-axis). Usually both axes are continuous or count data. Line graphs connect all the points, so this type of graph is only suitable when we are interested in the fine details of how our data move up and down. Scatter plots should be used when we are more interested in general trends. Also note that line graphs only work when there is just one yvalue associated with each x-value (scatter plots can have more than one y-value associated with each x-value). For example: 20 Weight of honey (kg) 18 16 14 12 10 8 6 4 2 0 0 50 100 150 200 250 300 350 Number of days since 1st January Figure 4. Weight of honey in one beehive in Sewanee, TN, plotted against time. 400 41 Note that we chose to have the y-axis start at zero. This is to emphasize that the honey supply never reached this point. It is sometimes appropriate to have a y-axis that does not start at zero, but if you do this, make note in the title, or in a legend, that the axes do not start at zero. Relative proportion in forest Bar graphs: So far we have only encountered graphs that are used with continuous or count data. In contrast to scatterplots and linegraphs, bar graphs are most useful when our data in divided into categories. The x-axis on a bar graph is divided into discrete categories, the y axis is usually continuous. For example, if we had measured the relative proportions of five tree species at one site in Sewanee we could plot the following graph: 0.35 0.3 0.25 0.2 0.15 0.1 0.05 0 White Oak Chestnut Oak Pignut Hickory Tulip Poplar Red Maple Species Figure 5. Relative proportions of five tree species at one site on the Sewanee Domain. Bar graphs can also be used to show standard deviations. For example, if we had repeated our measure of relative proportions at ten sites on the Sewanee Domain and found the following means and standard deviations: Table 3. Average and standard errors of the relative proportions of five tree species sampled on ten plots on the University of the South Domain. Species White Oak Chestnut Oak Pignut Hickory Tulip Poplar Red Maple We could draw the following bar graph: Mean Standard error 0.1 0.25 0.2 0.25 0.2 0.01 0.03 0.02 0.05 0.02 Relative proportion in forest 42 0.35 0.3 0.25 0.2 0.15 0.1 0.05 0 White Oak Chestnut Oak Pignut Hickory Tulip Poplar Red Maple Species Figure 6. Mean and standard deviation of the relative proportions of five tree species measured at ten plots on the Sewanee domain. ASSIGNMENT 1. The following graph is full of errors! The graph shows the mean percentage of “slow twitch” muscle fibers in the leg muscles of various athletes. The mean was taken from ten athletes in each category. % Muscle vs. Sport type 90 80 70 60 50 40 30 20 10 0 Swimmer Long-distance runner Sprinter Cross-country skier Weight lifter Sport On a separate piece of paper answer the following questions: i. Title: What is wrong with this graph’s legend? Suggest a better title. ii. 43 Axis labels: What is wrong with the labels on the axes? Suggest better axis labels. iii. Legend (the box on the right hand side): What is wrong with this graph’s legend? iv. Graph type: Why is this line graph inappropriate? 2. For each of the following situations, pick the most appropriate graph type (e.g., box plot, line graph, or scatter graph). Explain your choice in each situation. i. You have measured the abundance of ten species of bird in ten forest plots over 10 years. You want to produce one graph that will summarize how bird populations have changed through time. ii. You have calculated the average and standard deviation of the fat content of three brands of donuts. You want to summarize this data on one graph. iii. You have measured the body size of one hundred adult female darters (darters are fish that live in many Tennessee streams). You also counted the number of eggs in each female’s ovaries. How would you present the relationship between these two variables? iv. You have measured the membrane potential of an egg cell before, during and after entry by a sperm cell. How would you show how the potential changed through time? 45 CHAPTER 8: AN INTRODUCTION TO MICROSOFT EXCEL 2000 This tutorial will introduce you to data analysis and graphing in Microsoft Excel. You should work through this tutorial with Excel open on a computer so that you can work the examples as you move through the tutorial. How to enter data in Excel When you open Excel you should see a blank worksheet, which looks like this: Illustration 1. Blank excel worksheet. The worksheet consists of rows and columns of worksheet spaces, or "cells". Each row is numbered and each column has a letter. Every cell in the worksheet can therefore be referred to by a letter and number. For example, the top left hand cell is called A1, the cell below it is A2, etc. You can type either numbers or words in each cell. Imagine that over one week in July we had collected data on the maximum daily temperature in three locations (inside Woods Lab, on the lawn in front of the library and in Abbo's Alley). To enter this data into Excel, point the cursor on the cell, then click to select the cell. You can now type into the cell. In cell A1 type "Day". In cells B1, C1 and D1 type "Woods Lab", "Library Lawn" and "Abbo's Alley". Your worksheet should look like this: 46 Illustration 2. Sample worksheet showing category titles for a new table. Now you can enter the data into each column. Type the following numbers: Illustration 3. Sample data table. You can now save your work by selecting “Save” or “Save as” from the “File” menu. You can change the appearance of your worksheet by adding lines and by putting words or numbers in bold or italics. For example, click on cell A1 and hold the mouse button down. Now move the mouse over to D1 and release the mouse button. Cells A1, B1, C1 and D1 should all be selected. You can now click on the "B" button on the Formatting toolbar to make them bold (or the "I" button for italics, or the "U" button for underline): Illustration 4. Using the 'Bold' button on the formatting toolbar. You will notice that by making your column headings bold you have made the words slightly wider so that they no longer fit into their columns. You can correct this by 47 positioning the cursor so that it falls on the dividing line between the columns. The cursor should change from a fat cross to a skinny cross with two arrows. You can now hold down the mouse button and move the edge of the column. Alternatively, you can select your whole worksheet, then go to the "Format" menu and choose "Column", then "Auto Fit". This will automatically make each column just as wide as its widest cell. To add a line underneath the column headings, select the four heading cells (as you did to make them bold). On the Formatting toolbar select the Borders tool which looks like this: Illustration 5. Using the 'border' button to apply borders to a table. You can now add a line or a box around your text. Here is my data table with bold column headings, AutoFitted widths and a double line under the headings: Illustration 6. Sample data table showing data and all formats applied so far (bold column headings, autofitted widths and a double line under headings). 48 How to do simple calculations with data in Excel In this section you will learn how to calculate averages and do simple mathematical manipulations of your data. Calculating averages: Imagine that we wanted to compare the mean temperature for the three locations. We will put this information in cells B10, C10 and D10. Click on B10 then pull down the "Insert" menu and choose "Function…". The following box should pop up: Illustration 7. 'Paste function' box showing some function categories and functions. To calculate the mean click on "AVERAGE" in the right hand box, then click "OK". [Note: to calculate the median, click on MEDIAN, to calculate the mode, click on MODE]. A new box will pop up (if the box is blocking your table you can click on the top of the box, hold the mouse button down and drag the box to one side). In this new box you will tell the computer which cells you wish to have averaged. The temperature data for Woods Lab are in (B2 through B8 so type "B2:B8", then choose "OK". 49 Illustration 8. Function box for calculating the average of a range of values. If everything went well, you should have "20.142857" in cell B8. This is the mean of the seven temperatures for Woods Lab. Excel makes it very easy to calculate the means for our other columns. Click on cell B10 again, then position the cursor so that it is just above the bottom right hand corner of the cell. The cursor will change from a fat cross to a skinny cross. Now click the mouse button down and drag it across C10 and D10, then let go. You should see two new numbers in C10 and D10 - these are the averages of the other two columns. By using the skinny cross you have pasted the function "average" from cell B10 into cells C10 and D10. You can check that Excel did this correctly by clicking on C10. Above the worksheet you will see a little window that should contain the correct formula [=AVERAGE(C2:C8)]. The mean gives us a measure of the "center" of the data. We also want to summarize the variability in our data. One measure of variability is the standard deviation. Variable datasets have large standard deviations, uniform datasets have small standard deviations. To calculate the standard deviation in Excel follow the same directions as the calculation of the means: Click on cell B11, choose "Function" from the Insert menu and ask Excel to paste "STDEV", then type "B2:B8" to tell Excel where to find the data. You can use the skinny cross again to paste your formula into the adjacent cells. If all went well you should have the following values in your table (note that I have added labels and a line to make my worksheet clear): Illustration 9. Output table showing values for calculations of mean and standard deviations for temperature classes. 50 The means and standard deviations that Excel has given us go to eight decimal places, but our data were not measured this precisely! To change the number of decimal places, select B10, C10, D10, B11, C11 and D11 (to do this click on B10, hold the mouse button down and move over to D11) then choose "Cells" from the "Format" menu. The following box will pop up: Illustration 10. 'Format cells' option box. Click on "Number" in the left hand box, then use the scroll arrows to tell Excel to use just one decimal place, then click "OK": Illustration 11. Applying format of 1 decimal place to selected cells. 51 Your worksheet should now look like this: Illustration 12. Sample data table showing all formatting and calculations so far. To print this table, select the area you wish to print (cells A1 through D11), then go to the File menu, choose "Print Area" and "Set print area". You can now print what you have in the selected region. Now you will use Excel to create a new column using a simple mathematical manipulation of the other columns. Suppose that we wanted to know what the temperature difference was between the inside of Woods Lab and the Library lawn. Click on cell F2, then type "=C2-B2" into cell F2, then before pressing Return (or Enter) move the cursor to the bottom right corner of the cell until you see the skinny cross. Now click on this corner and pull down until you reach cell F8. When you let go of the mouse Excel will have calculated the difference between column C and column B for each row. Again, you should add a column heading to make your table clear: Illustration 13. Data table with heading for new column. Using the Fill function Excel can do many other types of mathematical manipulations of your data. Here is one more example: Suppose that we were studying some fish in a lake. The initial size of the fish 52 populations is 15 and the fish population doubles each year. We will use Excel to calculate the size of the population after 10 years. In cell A13 type "Year", in cell B13 type "Population size". Now type "1" in cell A14 and "15" in cell B14. You are now ready to make Excel do some work for you -- type "=A14+1" in cell A15, then press return. The number "2" should appear in cell A15. Now position the cursor over the bottom right corner of the cell until you see the skinny cross. Now click on this corner and pull down until you reach cell A23. When you let go of the mouse Excel will have produced a series of years from 1 to 10 by adding one to the cell above every cell in the column. Now click on cell B15 and type "=B14*2", then press return. B15 should now have "30". Click on B15 and position the cursor over the bottom right corner of the cell until you see the skinny cross. Now click on this corner and pull down until you reach cell B23. What is the population size of fish in year 10? You should have 7680. How to draw graphs in Excel Excel allows you to draw many different types of graph. Unfortunately, Excel is not smart enough to know which graph is the most appropriate for the type of data you have, so beware! Think about what kind of graph you draw before you start clicking away in Excel. You will now make a line graph of the temperature in the three locations throughout the week. First, select cells A1 through D8. This will highlight all the data and the column headings. Now go to the top of the screen and click on the "Chart Wizard": Illustration 14. 'Chart wizard' button on 'Standard' toolbar. A new box will pop up and give you some options. You will choose the XY scatter option on the left and the "connected data points" option on the right. Note: even though you are drawing a linegraph do NOT use the "Line" option! 53 Illustration 15. 'Chart type' selection box in 'Chart wizard' function of Excel. Click on "Next". The next box allows you to tell Excel where to find the data for the graphs. We have already selected our columns, so you can just click "Next" again. In the next box you will add axis labels, graph titles, legends, etc. Normally each axis on a graph should include a label with units. Type "Temperature (Degrees Centigrade)" for the y axis and "Day" for the x axis. Graph legends should explain exactly what the graph is showing and should include a figure number: "Figure 1. Graph of the maximum daily temperature during one week in July for three locations in Sewanee" would be appropriate for this graph. 54 Figure 1. Graph of the maximum Figure 1. Graph of the maximum daily temperature during one week in July for three locations in Sewanee. Illustration 16. 'Chart wizard' window showing options for chart formatting. Before clicking "Next", click on some of the other options to see how you can modify your graph (e.g., remove gridlines, change the location of the legend). Now press "Next" to go on to the last box. This box allows you to tell Excel where to put your new graph. You can choose "As an object" to have the graph appear alongside your data table, or "As a new sheet" to add the graph as another "sheet" in Excel. Using "sheets" allows you to keep your file tidy. Illustration 17. 'Chart wizard' chart location window. 55 You can switch between sheets using the tabs at the bottom of the screen (click on the tabs to move between sheets): Illustration 18. 'Page tabs' allowing you to move between pages of your 'work book'. You should now have a nice line graph of your data. If you want to change your graph just double-click on the part that you wish to change. For example, double-click on the y axis label to change the label text or font, double-click on the numbers on the y axis to change the range, double-click on the points to change their size or the thickness of the line. Now you will draw a scatter plot of the temperature in Abbo's Alley plotted against the temperature outside the library. To do this, first select cells C1 through D8: Illustration 19. Selecting cells to make a scatter plot. Now click on the chart wizard and select XY Scatter with no line: Illustration 20. Selecting xy (scatter) in chart wizard. 56 The rest of the process is very similar to our first graph. You should end up with a graph similar to the following: Figure 1. Graph of the temperature at Abbo’s Alley plotted against the temperature at the library lawn for seven days in July. Figure 7. Example of what your scatter graph should look like. The last type of graph that we will explore in this tutorial is the bar graph. We will plot a graph of the average temperature at the three locations. First select the cells that can contain the average temperatures: Illustration 21. Selecting cells to make a bar graph. Now click on the chart wizard and select "Column": Illustration 22. Selecting 'column' chart type and first subtype in 'chart wizard'. 57 In the next box leave the "Data range" as it appears (you selected your data, so Excel knows where to find it) and click on "Series": Illustration 23. Selecting 'series' tab in chart wizard. You will now tell Excel where to get the labels for the x axis. Click in the X axis labels box: Illustration 24. Selecting location of axis labels. Now click on cell B1 in your worksheet and hold the mouse down. Move the mouse over the cell D1. These will give Excel the labels for the x axis. Don't panic if part of your graph wizard disappears for a moment! You should see the following: Illustration 25. Location and range values for cells selected for axis labels. Click "next" to go on and add a title and axes labels to your graph. It should look like this when you are finished: 58 Figure 2. Graph of mean temperatures during one week in July at three locations in Sewanee. Figure 8. Example of properly formatted bar graph. You have one more step: adding symbols to your graph to indicate the standard deviations. You cannot do this in the chart wizard, only afterwards. Note that you can use the same sequence of clicks to put standard deviations on other graphs such as linegraphs. Click on your graph so that a dot appears in each column (if this doesn't happen, try clicking somewhere else on your sheet or graph, then clicking on the columns again): Illustration 26. View of bar graph with columns selected. Now double-click one of the columns. You will get a box with several options; choose the "Y error bars" option: 59 Illustration 27. Options available for column formatting. Now click on the "Both" box and the "Custom" box, then click in the box to the right of the "+" sign, then click on cell B11 in your worksheet and hold the mouse down. Move the mouse over the cell D!1. These will give Excel the values for standard deviation. Don't panic if part of your graph wizard disappears for a moment! Repeat for the "-" box, then recheck to make sure that "Both" is selected at the top of the options box. You should see the following: Illustration 28. Error bar formatting window. 60 Your completed graph should look like this: Figure 2. Graph of means and standard errors for temperatures during one week in July at three locations in Sewanee 35.0 30.0 25.0 20.0 15.0 10.0 5.0 0.0 Woods Lab Library Lawn Abbo's Alley Location Figure 9. Completed bar graph with proper formatting, labeling and error bars. 61 ASSIGNMENT: The data for this assignment are found on the Angelnet file server in an Excel 2000 workbook called "Sample data for Intro Bio". You should copy this file onto a disk and work through the following problems. To navigate to this file, choose the appropriate directions below. Mac-- Open the chooser from the Apple menu on the computer. You should see something like this: Illustration 29. View of the 'chooser' window where the AngelNet file server can be accessed. Click on AppleShare in the top left box, then Sewanee Net Servers in the bottom left box, then 00-AngelNetFileServer in the top right, then click OK. A box will pop up - click on the "Guest" button. The following will come up: 62 Illustration 30. Menu of items found on the AngelNet file server. Click on "Acad_Classes" then click OK. The following icon should appear on the desktop of your computer: Illustration 31. 'Academic classes' icon that will appear on your computers desktop. Double-click on this, then open the following folders: Sciences Math/Biology/Intro Bio/Stats. The file "Sample data for Intro Bio" is in this folder. To make a copy of this file, drag it into a folder on a disk. Now disconnect from the Academic Classes on Angelnet fileserver by dragging its icon into the trash. PC—Open the ‘Start’ menu at the bottom left corner of your desktop with a single click, choose ‘Run’ from that pop up menu. In the ‘Run’ dialog box type \\angelnet_server\Acad_Classes then press ‘OK’. A window titled ‘Acad_classes on angelnet_server’ will appear on your desktop with several folders inside. Navigate through the following folders: Sciences Math/Biology/Intro Bio/Stats. The file "Sample data for Intro Bio" is in this folder. To make a copy of this file, drag it into a folder on a disk. Now disconnect from the Academic Classes on Angelnet fileserver by clicking on the ‘X’ in the upper right corner of the window or chose ‘Close’ from the ‘File’ pulldown menu. 63 Open the file ‘Sample data for Intro Bio’ in Excel and answer the following questions on a separate sheet of paper: 1. The “Farms” column has data about the size of dairy cow herds on farms in Tennessee. Each number represents the number of cows on a farm. There are 30 farms in all. i. What is the mean and median number of cows on each farm? ii. Why do the mean and median differ? iii. Would you report the median, mode or mean, if you wanted half the farms in the sample to be above the value and half below? 2. The "Volume" and "Weight" columns contain data about the volume and weight of 40 hickory nuts that were collected in the fall of 1998 in a cove forest in Sewanee. Draw an appropriate graph to show whether volume and weight of these hickory nuts are correlated with each other. Make sure you fully label your graph, then print it out. 3. The next table shows data about performance scores for five pigeons. These birds were trained to perform a complex series of pecks with their beaks in order to receive a reward. Their "score" summarizes their performance in this task. Scores are shown for each pigeon on each of ten days. There are two blank columns: mean and standard deviation. You should use Excel to calculate these means and standard deviations, then plot a suitable graph to show how the scores changed through time. Include the standard deviations on your graph, Print out this graph. 4. The "Year" and "Human Population" table shows the estimated human population over the last 1000 years. Plot a suitable graph to show this data. How long does it take for the human population to double? If past trends continue, what year will the populations hit 12 billion? 65 CHAPTER 9: USING EXCEL TO PLOT DATA (BIOLOGY 132) A. GETTING SET UP • • • • • • Turn on computers Login (should be same as email login) Find Excel and open it Under the Help menu, make sure that the Microsoft Assistant (that annoying animated paper clip) is turned Off. If there is an option that says “Turn Assistant Off”, scroll down and highlight this. If the option reads “Turn Assistant On” then it is already turned off. Under the View menu > Toolbars, make sure that the Standard and Formatting toolbars have a check (√) beside them so that they will be displayed at the top of the screen. Under the View menu make sure that the Formula Bar has a check beside it so that it will be displayed. A blank page should appear on your screen • this is a worksheet where you can enter your data • each rectangle in the worksheet is called a cell • to enter something in a cell, simply highlight the cell (click on it) and begin typing • use tab key to move across the table, return key to move down the table, mouse to click on a cell and move to it. You can also use the arrow keys to navigate among cells B. ENTERING YOUR DATA • • • • we will begin by entering your spectrophotometry data from last week set up a data table like you did in your lab notebook, with one column headed Wavelength (nm), and the second column headed Absorbance enter your data 420,440, 460 etc. down to the final wavelength used, and include the measurements you made every 5 nm at the end, hitting return after each entry to advance down to the next cell in the column. Do the same for the corresponding absorbance readings (e.g. 0.148, 0.179. 0.210 …) Remember: you can always UNDO any changes you made by using the Edit > Undo command (CTRL Z on PCs, or ‘apple’ Z on Macs) C. SAVING YOUR WORK • • File>Save> “filename” before pressing ok, designate where you want to save it on the computer. By default it usually puts it in My Documents, or something like this. To make it easy to find, save it to the Desktop. You can always move it later if you want to. D. FORMATTING YOUR DATA • Note that you will need to SORT your data in order of increasing time to get Excel to graph it properly. To do this, highlight all of the values in both columns, and from • • 66 the menu at the top select Data > Sort.... From the pop-up menu, choose the column you wish to use as your sorting category and whether you want values in ascending or descending order (choose ascending). Click OK and check to see that the entire area you wanted sorted is highlighted, and that things have sorted the way you want. use the Formatting toolbar to format text to your liking (e.g. centering text, or right/left justify text in columns, changing font size, bolding/underlining text, adding borders) adjust column width and row height by placing the cursor between at the end of the column or row you wish to adjust, hold down the mouse button and drag to the desired size. To automatically resize a column or row, place the cursor at the end of the row or column and double-click on the separator. E. GRAPHING YOUR DATA By default, Excel graphs data by using the left-hand column for the X axis, and the righthand column for the Y axis. Setting up your tables with this in mind makes it relatively easy to produce a chart (= graph = figure) in Excel. • using your wavelength/absorbance data, highlight the complete data set, including the column headings (Wavelength , Absorbance). To do this, click on the top left cell (Wavelength), hold the mouse button down and dragging it over the entire area, to the bottom right corner. • release the mouse button. The entire data set should now be highlighted. • click on the Chart Wizard icon in the Standard Toolbar and a menu will appear • STEP 1 - CHART TYPE: pick the type of graph you want to make. If you are not sure, select one and click on the Press and hold to view sample button to preview the graph. You can use this feature to explore different ways of presenting your data. • what type of data do you have? Column, Bar and Line charts use discrete data (categories or counts) on the X-axis and continuous data on the Y-axis; Scatterplots use continuous data on both the X- and Y-axes. • once you select graph type, Excel will take you through a series of steps in building the graph. You can go back or bail out at any point. After you have pressed Finish, you can still edit the graph. • STEP 2 - CHART SOURCE DATA: if you highlighted your complete data table before you hit the Chart Wizard tool, all should be fine. If not, you can make adjustments here. • STEP 3- CHART OPTIONS: o Titles: Chart title: Use this for your Figure title: E.g. Figure 1. Spectrophotometric determination of … (you fill in the rest) Value (X) axis: enter the independent variable name (and units if applicable) Value (Y) axis: enter the dependent variable name (and units if applicable) o Axes: both X and Y value boxes should be checked 67 • • • o Gridlines: a check in a box displays the gridlines noted. Default is Y-axis major gridlines on. Some prefer to turn these off. turning on X- and Y-axis major gridlines will give the appearance of graph paper, which can be useful if you are trying to use the graph to scale off values. o Legend: Default is “Show Legend” This is only necessary if you have more than one dependent variable. For the first graph today, turn it off, for the second graph, you will need to have it on. o Data labels: Default is none – keep it like that STEP 4- CHART LOCATION: here you have the option of placing the chart on the same page as your data (as object in the current worksheet – this is the default option), or placing the chart on a separate sheet. for starters, place the chart on a separate sheet (you can always move it later). This way you get a full page blowup of your chart and it is easier to format It helps to name the sheet so that you can find it easily later (e.g. change Chart 1 to an informative name – e.g. WavMaxChart). The name will appear on the tab at the bottom of the worksheet. When you click FINISH the chart will appear where you asked to place it. Now you can continue to format the chart. F. FORMATTING YOUR CHART Double-clicking on many areas of the chart will bring up a menu that gives you various options. For example Getting rid of the gray background on the chart (it only wastes toner or ink) • double click on the background of the graph (somewhere within the axes) to change the background color and plot frame (Default is gray with a gray border). Choose Patterns > Border > None, and Patterns > Area > None • the same menu can be obtained by a single click on graph background, then going to the Format menu at the top > selected plot area Rescaling X- or Y- axis • double click on the axis of interest • enter the most appropriate minimum and maximum values and interval units (e.g. Wavelength 400-700 nm, interval scale will depend on the size of your graph) Editing and moving chart titles and axis labels • single-click on the label of interest and a box will appear around it. You can then drag the box to move it to a new location. ALWAYS MOVE YOUR FIGURE TITLES TO THE BOTTOM OF THE FIGURE • after the box appears, a second single-click will allow you to edit the text in the box 68 • • • double-click on the label to bring up a menu box that allows you to change font size, color, etc. selected text can also be reformatted using the formatting toolbar To change font or font size for the entire chart, click in upper left of chart (or anywhere outside of the axes). A black square appears around the chart to indicate that the entire chart is selected. Then use the formatting toolbar to select the desired font features that will be applied to the entire chart (alternatively, go to the Format > selected chart area > Font tab from the menu bar at the top of the screen) Edit Chart features using the Chart menu from the top of the screen • the menus you saw when you were first constructing your chart are found under the Chart menu at the top of the screen. This tab is only visible when the active cell selected by your mouse is within the chart area (i.e. you won’t see it if your cursor is in a data cell of a worksheet). From these you can change your chart type, add new series of data, edit chart features, and move the chart to a different location. SAVE YOUR WORK! G. PREVIEWING AND PRINTING YOUR CHARTS • • • preview your chart before printing by selecting File > Print Preview. The chart will only appear if your cursor has selected the chart or sheet as the active area In page setup (or the Setup button from the Print Preview screen), you can change the page orientation (landscape vs. portrait), set the page margins, add headers or footers (usually not necessary here since your figure will have a title) and define how you want your Chart to be sized. when you are satisfied with your print preview, select PRINT H. MORE THAN ONE DEPENDENT VARIABLE – PLOTTING A DOUBLE Y-AXIS In your second experiment, in which you measured absorbance and % transmittance for each of five different dilutions of neutral red, you have 2 dependent variables (absorbance and % transmittance) • enter and save your data into a new Excel workbook • This time, you should have 3 columns of data: Concentration of neutral red (mg/ml), Absorbance, % Transmittance • • • • highlight all the data, and select the chart icon Select chart type (continuous data, therefore scatter plot) go through steps until you get the chart place the chart on its new sheet Notice: both variables appear, but you can barely see the absorbance data. This is because they are both plotted on the same Y axis, where the scale runs (at present) from 0-120. It 69 would be much more useful if the absorbance was on a scale from 0-1 (or 0-just above your max. absorbance reading) and % transmittance was on a scale of 0-100%. To put the two variables on separate axes, but within the same graph, one variable needs to go on a ‘secondary y axis’, which will be labeled on the right-hand side of the graph. • • • • • • • • • To change the axis for one of the variables, double click anywhere on the data line for that variable (% Transmittance is easier since it is more accessible). a menu appears that has several tabs. Click on the ‘axis’ tab. It is telling you that for the line you selected (%T) it is being plotted on the primary (left hand side) axis. click on secondary axis to shift %T over to the secondary axis. note in the graph preview that the absorbance now covers almost the entire height of the graph. This is because the primary y axis has been rescaled automatically to best fit the absorbance data. click okay and have a look at your newly formatted graph. rescale the y axes to get absorbance 0-1 (or whatever is most appropriate given your absorbance readings), and %T 0-100 % do any other formatting changes that you want. MAKE SURE YOU GIVE YOUR FIGURE AN APPROPRIATE TITLE preview your chart check with your lab instructor before printing your work I. FINDING THE SLOPE OF THE LINEAR PORTION OF THE CURVE In the enzyme exercises and your enzyme experiment, you will need to determine the slope of the best fit line through the linear portion of your data. This will give you the rate of enzyme activity. You will first graph all your data and print it. Use that graph to determine which part looks the most linear. Here’s how you get the rate over the linear part of the curve. • select only the cells in all columns corresponding to the range of times where the data appear linear and copy them. • click on your graph anywhere • from the Edit menu choose Paste Special and within that click on Categories (X values) in First Column and then click OK. • click on a data point in the linear portion of the graph (should be a different color from the other points) • from the Chart menu choose Add Trendline • click on the Type tab • click on Linear • under Based on Series in that same window, choose the series for the trendline • click on the Options tab • place check marks in Display Equation on Chart • click OK • repeat from Add Trendline until you have trendlines for each of your reactions • the formula for the line is y = mx + b and so “m” is the slope = rate of reaction 71 CHAPTER 10: INFERENTIAL STATISTICS OR "STATISTICAL TESTS" We use inferential statistics when we want to go further than simply summarizing our data. For example: (i) we might want to use some data about trends in the abundance of an endangered species to predict whether the species will decline or whether it might increase, (ii) we might want to test whether one brand of insect repellent is more effective than another. These two examples illustrate the two main types of statistical test: those that look for a difference between two groups of numbers (e.g., the number of insects biting your arm) and those that look for an association or correlation between groups of numbers (e.g., the abundance of an endangered species and time). Why use statistics when we can just show our data on a graph? Statistics allow us to make an informed decision about whether the patterns we see in our data are due to chance or not. For example, if we toss a coin 10 times and get 6 heads and 4 tails, we would not be inclined to suggest that the coin was “unfair”, whereas if we got 10 heads and no tails, we might be suspicious. How about an 8:2 ratio, or a 7:3? Statistics provides rules to help us decide in these borderline cases (and most data are “borderline”, at least in the eyes of reviewers of scientific manuscripts!). Statistical tests will not provide definitive answers - for example, tossing a coin and getting twenty heads in a row indicates that the coin might be double-headed, but no statistical test can tell us for sure. What statistical tests can do is provide us with a quantitative evaluation of the probability that the patterns we see in our data are due to chance alone. For example, a statistical test on our coin-tossing data would tell us that if we sat down and tossed a coin 20 times, then repeated this a million times, that on average only one of the million trials would give us 20 heads in a row. We would say that the p-value (p for “probability”) for getting 20 heads in a row is about p = 0.0000001. Thus, the output of most statistical tests will be a probability usually called the "p-value". The p-value is a measure of the probability that the pattern we see in our data is due to chance alone. That was a pretty extreme example. Most of our data give us much larger p-values. For example, suppose we conducted a statistical test to see whether a random sample of Dunkin’ Donuts had more fat in them than a random sample of Mister Donuts (we’ll learn how to actually do these tests in a moment). The mean fat content for Dunkin’ Donuts was 5.5 grams and 4.9 grams for Mister Donut; the statistical test gives us a p-value of p=0.02. What does this mean? The p value tells us: "If Dunkin’ Donuts and Mister Donut did not differ in their fat content we would expect samples from each of them to differ by this amount (0.6 grams) in just 2% of cases". Is a 2% chance (i.e. p = 0.02) small enough to conclude that Dunkin’ Donuts are more fatty? 72 In general, if the p-value is less than 0.05, we conclude that we have found a “statistically significant” result. NOTE: This 0.05 (or 5%, or 1 in 20) is an arbitrary cutoff point. If the cost of making an incorrect decision was very high, we might want to chose a cutoff off 0.01 or even 0.001. So that explains the magical p-value. We’ll see now how these p-values fit in with scientific hypothesis-testing: Two fundamental ideas in science and in statistics are the null hypothesis and the alternative hypothesis. For any statistical test we should specify both. The null hypothesis (sometimes referred to as Ho) describes the situation where there is no difference or association within our data. The alternative hypothesis (sometimes referred to as HA) is that there is some difference or association in our data. For example, if we were to compare the length of beaks on male and female downy woodpeckers Ho and HA would be as follows: Ho: Male and female downy woodpeckers have the same length beaks. HA: Male and female downy woodpeckers have beaks of different lengths. Alternatively, if we conducted a more detailed statistical test we might state multiple alternatives: Ho: Male and female downy woodpeckers have the same length beaks. HA1: Male downy woodpeckers have longer beaks than female downy woodpeckers. HA2: Female downy woodpeckers have longer beaks than male downy woodpeckers. We can now rephrase our understanding of the p-value in terms of null and alternative hypotheses. First, we acknowledge that even if the null hypothesis is true, chance alone will sometimes produce data samples that contain some pattern in them (e.g., tossing a coin and getting 20 heads in a row). The p-value is the probability of finding this pattern in our data if the null hypothesis is true. Let’s revisit the donut example and state it in terms of null and alternative hypotheses: Ho: Dunkin’ Donuts and Mister Donut have the same fat content. HA1: Dunkin’ Donuts has a higher fat content than Mister Donut. HA2: Dunkin’ Donuts has a lower fat content than Mister Donut. We would state our conclusion as follows: “We reject the null hypothesis and accept the alternative hypothesis (at the p=0.02 level) that Dunkin’ Donuts has a higher fat content than Mister Donut.” If the p-value had been p=0.13, we would state: “We do not reject the null hypothesis (p=0.13) that Dunkin’ Donuts and Mister Donut have the same fat content”. 73 NOTE: ◊ Always either reject or do not reject the null hypothesis. Never reject or fail to reject the alternative. ◊ Always report your p-value when making statements about rejecting or not rejecting the null. ◊ You can never prove the alternative hypothesis. At best you can “accept” or “support” it at a given p-value. If you are at all familiar with Karl Popper’s views on how science should be conducted you will see some strong parallels here: Science moves along by falsifying hypotheses (i.e. rejecting null hypotheses), and nothing is ever proven in science (i.e. we never prove the alternative hypothesis). We'll now explore two different statistical tests and you'll learn how to do these tests in Excel 2000. First you need to tell Excel 2000 to load up a package of "add ins" that will allow it to conduct statistical tests. To do this, go to the "Tools" menu and select "Add-Ins". A box should pop up - click on "Analysis Tool pack" so that a check mark appears to its left then click OK (if the check mark is already there you can just click OK). The following examples will use data that is in the file "Stats examples for Intro Bio" that is in the same folder on the FileServer as the sample data you used for your previous worksheet. You should copy this file onto a disk, and follow along See the worksheet at the end of Chapter 9 in your Survival Guide for detailed instructions to retrieve the data file. Launch Microsoft Excel and open the file ‘Stats examples for Intro Bio’. The t-test: Use a t-test to compare the means of two samples. In order to use this test your data should be continuous, but sometimes people use this test with count data (i.e. integers). We will use the first two columns for our t-test. These columns show data on the average width of the annual growth rings from chestnut oaks growing on the bluff (i.e. on the very edge of the cliff) and on the interior of the Plateau. Each number represents data taken from one tree, so 30 trees were sampled in each group. We want to know whether the means of these two groups of numbers differ significantly from one another so we will use a t-test. Thus, our null and alternative hypotheses are: Ho: Bluff and interior plateau chestnut oaks have the same mean width of their annual growth bars. HA: Bluff and interior plateau chestnut oaks differ in the mean width of their annual growth bars. Go to the "Tools" menu and select "Data analysis", then choose "t-test: Two-sample assuming equal variances" as shown below: 74 Illustration 32. Data analysis 'tools' window. *** If 'Data Analysis' does not appear under your 'Tools' menu then do the following: Under 'Tools' choose 'Add-Ins', check (√) 'Analysis Tool-Pack' then select OK. When your computer stops buzzing you should be able to find 'Data Analysis' on the 'Tools' pull-down menu. Excel 2000 will now ask you to fill in several boxes. For the "Variable 1 range" you should click in the box to the right of the text, then select all the numbers in the "Bluff" column: 75 Illustration 33. T-test box and data sheet, showing selection of cells for variable ranges. Now click in the Variable 2 range box and select all the numbers in the Plateau column. For "Hypothesized mean difference" type in 0 (zero). We want to test whether the means differ from each other, so our null hypothesis is that the means are the same. For "Alpha" type in 0.05. This is the cutoff point for our p-value - if it is below 0.05 we will conclude that there is a significant difference between the means. Last, click on "New worksheet ply" and type in a name for this sheet. This will put your results on a new sheet in your Excel 2000 workbook. 76 When you are all done your box should look like this: Illustration 34. T-test box with variable ranges selected. Press OK and Excel 2000 will conduct the test for you. Your output will look like this: Illustration 35. Output table following t-test. 77 What does all this mean? The most important row in the table is: "P(T<=t) two-tail". This gives the p-value for this test. Here p = 0.0032 The other rows also have some interesting data: The first row shows the mean value of your two data columns. Thus, the Bluff data have a mean of 0.406, the Plateau data have a mean of 0.617. The "Variance" row gives the variance which is a measure of the amount of variation in the data (variance = (standard deviation)2). The "Observations" row shows the sample size in each sample. The "df" row gives a number called the "degrees of freedom". This number is used in the statistical test as a measure of the sample size. For technical reasons, df is always lower than the actual sample size. That is a lot of information. How should you report this statistical test in your lab reports? Here is one way: A t-test indicated that there was a significant difference between the means of the two groups (p = 0.0032, df = 58). These data therefore support the hypothesis that Chestnut oaks growing on the Bluff have narrower growth rings (mean = 0.404 mm) than Chestnut oaks that grow on the interior Plateau (mean = 0.616 mm). Note that: ◊ You should always give the p-value and the degrees of freedom. ◊ You never prove your hypothesis - you can just support it. ◊ You should give some indication about the magnitude of the effect you have studied (in this case we reported the actual means). If you have a large enough sample size you can detect very, very small differences between groups. These differences might not be biologically meaningful. Linear regression Use these if you want to use one set of numbers to look for an association between two groups of numbers or when you want to use one set of numbers to predict the value of another. Only use this test if a scatter plot of your data suggest that there might be a linear relationship. Here are some examples. ONLY graphs A and D suggest a linear relationship (although graph D might also be an S-shaped relationship). 78 A B * * * * * * * * * C * * * D * * * * * * * * * * * * * * * * * * * * * * * Illustration 36. Four scatter graphs illustrating potentially linear and non-linear data. Graphs A and D suggest potential linearity, while graphs B and C do not. Linear regression uses one variable (=independent variable, x axis) to predict the value of another (=dependent variable, y axis). The regression will calculate a “best fit” line through your data. This calculation involves minimizing the square of the distance between the line and each point (you don’t need to know the details of this calculation) - the line is therefore called the “least squares” line. NOTE: statistics software packages will calculate a linear regression through anything regardless of whether the data actually are linear. If your data are linear, the regression line allows you to predict the value of the variable on the y-axis from the value of the variable on the x-axis. If your data are not linear, the best fit line will give you garbage! The example for linear regression is also in the Stats Examples file. The data show measurements taken from 30 cardinal flowers (these are plants that grow throughout Sewanee - they are found in wet areas and have bright red flowers that are pollinated by hummingbirds). We have measured the mass of all the roots and the number of seeds produced by each plant. We want to know whether root mass can predict the number of seeds produced. Thus, our null and alternative hypotheses are: 79 Ho: The root mass of cardinal flowers does not predict the number of seeds produced by each plant. HA: The root mass of cardinal flowers predicts the number of seeds produced by each plant. [Note that this alternative hypothesis does not state whether there will be a positive or a negative relationship between root mass and number of seeds. We could write two alternative hypotheses: one for a positive relationship, one for a negative relationship] To conduct a regression: Select "Data Analysis" from the tools menu. Then choose "Regression". Fill in the Data Range boxes by clicking in them, then selecting the "Root mass" data for the x-axis and "Number of seeds" data from the y-axis. Type in a name for the new sheet for your results, then press OK. Illustration 37. Regression box, showing cells selected for x and y input ranges. Excel will then show you three tables that summarize the results of the regression. The p-value is given in the second table in the box at the far right hand (under "Significance F"). In this case p = 0.426. This is greater than 0.05, so we have not rejected the null hypothesis. The degrees of freedom are given in the second column of the second table. In this case we have 28 degrees of freedom. We could report these data by writing: A linear regression indicated that the root mass of cardinal flowers did not predict the number of seeds produced by each plant (p = 0.426, df = 28). These data therefore fail to reject the null hypothesis that there is no relationship between root mass and number of seeds produced. 80 The tables also give some other interesting information. The linear regression calculates the equation of the best straight line through the data. This equation has the form: y=mx+c where m is the slope of the line and c is the intercept on the y axis In this case, the intercept is 5.806 and the slope is -0.145 (these values are in the first column of the last table). We can get Excel 2000 to draw a scatterplot with this best fit line as follows: ¾ --Draw a scatterplot (remember to label all axes and give it a title) ¾ --When you have finished the scatterplot go to the "Chart" menu and select "Add trendline": Illustration 38. Trend line options found in the chart menu. ¾ Select "Linear", then click OK. Excel will draw a best fit line for you: Figure 1. The number of seeds produced by cardinal flowers plotted against the root mass of each plant. 12 10 8 6 4 2 0 0.00 2.00 4.00 6.00 8.00 Root mass (grams) Illustration 39. Completed scatter plot with best fit line. 10.00 12.00 81 Unfortunately there is a slight discrepancy between the formula used by Excel to draw this line and the formula used in the regression, so the equations for each are not exactly the same. They are very close, however. The equation from the regression table is the more accurate one. ASSIGNMENT: Answer the following questions on a separate piece of paper. 1. I have measured the mass of 5 male trout and 5 female trout. Is a t-test an appropriate way of evaluating the hypothesis that female trout have higher mass than do male trout? Explain why or why not. 2. I have conducted a linear regression of the body mass of rattlesnakes against their body temperature. My regression gave me a p-value of 0.04 and I had 50 rattlesnakes in my sample. Write down a null and alternative hypothesis for this regression and explain what this p-value means. 3. The "Stats examples for Intro Bio" has data on the concentration of sodium ions in samples of water taken from two rivers. We want to compare the means of these two groups of numbers. Write down a null and alternative hypothesis, conduct an appropriate statistical test and report for results as you would in the results section of a lab write up. You do not need to include a graph for this question. 4. The "Stats examples for Intro Bio" also has data on the area of individual leaves from a magnolia tree and the number of insects found on each leaf (i.e. each leaf was plucked from the tree, had its area measured and all the insects on each leaf were counted). We want to know whether leaf area can be used to predict the number of insects on each leaf. Write down a null and alternative hypothesis, conduct an appropriate statistical test and report for results as you would in the results section of a lab write up. Include a graph with your results. 83 CHAPTER 11: USE OF OXFORD® DIGITAL DIAL PIPETS Carefully following these steps will repay you with better results in your work. 1. Set the volume by turning the adjusting knob that is the top of the plunger. Do not turn the knob to beyond the maximum or minimum ranges listed on the top of the pipet. 2. Put a disposable plastic tip on the end of the pipet by inserting the bottom of the pipet into a tip in a rack. Do not use your hands to remove a tip and then put it on the pipet. 3. Secure the tip better by grabbing the top of the tip and pushing it on tighter while twisting. Do not touch the tip more than 2 cm from the top - if you do, you could introduce contamination into your solutions. 4. Push the plunger of the pipet down to the first stop position before putting the tip into the desired solution. If you were to insert the tip into the solution before doing this, you would blow bubbles out of the tip, and a small bubble of air would remain at the tip, preventing you from pipeting accurately. 5. Insert the bottom of the plastic tip just below (approx. 1 mm below) the surface of the solution you wish to withdraw, while holding the plunger at the first stop. The best way for a right-handed person to pipet is to hold the tube or bottle containing the solution with the left hand at eye level 4 to 6 inches from the face, so that the fluid can be seen clearly. Hold the pipet with the right hand. This procedure can even be used if you are making solutions on ice. Simply pick the tube up from the ice by holding it at the top (so that you are not heating the solution at the bottom much) and keep it off the ice as briefly as possible. 6. While holding the tip just below the surface of the solution, release the plunger slowly. Don't just let the plunger snap up. (If you were to allow the plunger to snap up the pipet will not draw the proper volume of fluid. This is critical for accurate pipeting of all solutions.) 7. Remove the tip from the solution. 8. While holding the tube that will receive the solution at eye level, put the tip of the pipetman into the recipient tube. 9. If the tube is empty, place the bottom of the tip along the side of the tube near the bottom. If the tube has some other solution in it already and you want to mix the solution being delivered, put the bottom of the tip just below the surface of the resident solution. 10. To eject the solution, press the plunger down. You will notice that when you reach the first stop on the plunger there will still be a small amount of solution in the tip. To expel this last amount, depress the plunger fully. 11. To prevent solution from re-entering the tip, you must remove the tip from the solution or away from the side of the tube while still fully depressing the plunger. If you do not do this, you will make the very common mistake of sucking solution up into your tip even well past the set volume. 84 12. Discard the tip by pressing the tip ejection button. Tips should be placed into the regular trash baskets. You may wish to collect used tips at your bench instead of running to the trash with each tip, but be sure to use a fresh tip for every operation. Practice Settings We have three sizes of digital dial pipets, each with color-coded dials on top: 0.5-10 µl (white), 10-100µl (yellow), and 100-1000µl (blue). They are referred to, respectively, as: P10, P-100, and P-1000. The color-coded dials indicate the size and color of protective plastic tips to be used with each. Volumes are dialed in using the colored knobs while reading the four digit setting in the window. If a window has a horizontal line through it, indicated in the window boxes below by a bolded line, you are to read a decimal at that line. NOTE: Pipets should never be dialed above or below the volumes indicated on the pipet. P-1000: to set 950 µl (has no decimal indicating line) 0 1000's of µl 9 100's of µl 5 10's of µl 0 1’s of µl P-100: to set 75 µl 0 100's of µl 7 10's of µl 5 1's of µl 0 0.1’s of µl P-10: to set 10.5 µl 1 10's of µl 0 1's of µl 5 0.1's of µl 0 0.01's of µl 85 Dial and Plunger Button Volume Setting Lock Tip Ejector Button 0 Volume Setting Window 1 2 5 Filter Figure 10. Representation of an Oxford® digital dial pipet. 87 CHAPTER 12: SPECTROPHOTOMETRY Many biological materials are colored (e.g., chlorophyll, melanin) or may be rendered colored by reaction with specific dyes (e.g., the Feulgen reaction for DNA). This fact enables biologists both to identify and quantitate such substances relatively easily. Identification is accomplished, or aided, by obtaining an absorption spectrum of the molecules in solution. This is possible because colored solutions do not absorb the energy of all light wavelengths equally. (The wavelengths of the visible spectrum are measured in nanometers [nm], spanning about 400 to 700 nm. One nm is 10-9 meters; see the preceding table on linear measures.) The chlorophylls, for example, absorb strongly in the blue wavelengths (ca. 450 nm) and red wavelengths (ca. 650 nm), but not in the green wavelengths (ca. 525 nm). Thus, plotting absorbance versus visible wavelengths for a solution of chlorophyll yields a line that appears as two mountains with a deep valley between. This topography, or absorption spectrum, is characteristic of chlorophyll and may be used as an aid in its identification. Quantitation is possible because at a given wavelength, absorption by a fixed thickness of a solution is proportional to the concentration of solute (Beer-Lambert Laws). Since small amounts of solute can be better detected at the wavelength where absorption is greatest (the absorption maximum), it is customary to use the absorption maximum for such quantitative determinations. A spectrophotometer consists of a light source, a device for selecting a specific wavelength, a “slit” through which a narrow beam of the desired wavelength passes, a solution holder (known as a colorimeter test tube, or cuvette), a photosensitive cell which measures the energy of light transmitted through the solution, and a recording device such as a galvanometer. A spectrophotometer must be calibrated at each wavelength so that the solvent of the solution absorbs no light energy (i.e., transmittance is 100%). A “blank” is used to calibrate the machine. The blank is a cuvette that contains everything except the specific compound for which the absorption is being determined. After “blanking”—i.e., setting the machine to read infinite absorbance when the cuvette holder is empty and 0% absorbance using the “blank”—the test solution is inserted, and the absorbance is noted. Percent transmittance is not proportional to solute concentration, but absorbance is proportional to solute concentration. The transmittance scale is linear and reads from left to right, whereas the absorbance scale is logarithmic and reads from right to left. One may read transmittance or absorbance directly (realizing the log nature of the latter). Using the Hach Odyssey DR/2500, the absorption for a solution can be obtained in two ways: 1. in 'Single Wavelength' mode the user can manually key in each wavelength, zero the machine using a blank, then measure absorbance of the colored solution. 2. in 'Wavelength Scan' mode, the user can have the machine automatically zero the blank over a range of wavelengths, and then measure absorbance of the solution over the same range. Both operating modes will be outlined below. 88 Manual determination of absorption using Single Wavelength mode 1. Turn on the spectrophotometer with the blue power button on the lower left side and let it equilibrate for 5 minutes. Select the 'Single Wavelength' function by pressing the appropriate button on the screen touch-pad. 2. To select a wavelength (e.g. 420 nm), select the lambda (λ, a symbol meaning wavelength) button on the touchpad, enter 4-2-0, and press “OK.” 3. Check the main screen to determine whether the spectrophotometer is set to measure absorbance (Abs) or percent transmittance (%T). The unit of measurement appears onscreen above the wavelength value; it is possible to toggle between these two measurement units by pressing the small button on the right, below the folder icon. 4. Slide the Odyssey cover back to reveal the test tube holder. Polish, then place the blank test tube (water only) into the test tube holder. Align the test tube so that the “F” emblem faces (an index mark) the exclamation mark on the test tube holder. Slide the cover forward to close. 5. Touch “ZERO” on the screen to set the absorbance reading of the blank to zero. Absorbance should now read 0.000 (the reading may fluctuate slightly). 6. Open the cover of the spectrophotometer and remove the blank. 7. Polish, then insert the tube containing the solution to be tested into the tube holder, aligning the index mark, close the cover and read absorbance. 8. Remove the tube and close the holder. 9. It is important that the instrument is calibrated or “blanked" each time the wavelength is changed. Automated determination of absorption maximum using Wavelength Scan mode 1. From the main menu select “Wavelength Scan”, then press the “Options” button to adjust wavelength range and absorbance scale. 2. Select “λ range” on the touchpad. For an example, you may choose to set the lower range to 420 nm by pressing the left on-screen button and entering 420 on the numberpad. Press “OK.” Set the upper wavelength to 620 by pressing the right onscreen button and following the same procedure. Your range screen should now display 420 nm – 620 nm. Press “OK.” 3. Select “Scale and Units” on the touchpad. Adjust the settings so that the screen Scale: ~ Auto. Press “OK.” appears as follows: Units: ~Abs 4. Press “Return” to get to the Wavelength Scan screen. 5. Insert the blank (water only) into the test tube holder and zero the machine (this will take approximately 60 seconds). 6. Remove the blank tube and insert the tube containing the solution to be tested. Press “Read” to begin the wavelength scan. 7. When the scan is complete, the machine will beep and display a graph of the results. To determine the wavelength maximum, use the arrow keys on the touchpad to move the vertical dashed line right or left, toward the highest point on the curve. Watch the highlighted absorbance reading (above the magnifying glass icon) as you scroll along the highest part of the curve and record the maximum absorbance reading – this may occur along at more than one wavelength. 89 CHAPTER 13: OSMOMETRY Principles of Function: Osmolality (osmoles per kg solution) and osmolarity (osmoles per liter water) are measures of the total concentration of dissolved particles in a solution, without regard for the homogeneity or non-homogeneity of the molecular species, or their molecular weights, size, or density. Any substance dissolved in a solvent affects four interrelated colligative properties of the solvent-solute mixture. These colligative properties are 1) decrease in vapor pressure, 2) decrease in freezing point, 3) increase in boiling point, and 4) a change in osmotic concentration. All of these properties are interrelated and are mathematically convertible. Therefore, the accurate determination of any one of these properties allows estimation of the other three, and is a measure of the osmotic concentration of the solution. Freezing point depression is one of the oldest and easiest methods for determination of the osmotic concentration of biological fluids. By this method, the specimen is placed in a cooling chamber which is maintained at a temperature well below the freezing point of the solution. During the analysis, samples are supercooled, at which time crystallization is initiated in a process called “seeding”. Seeding can be achieved by a variety of methods, and in this case is performed by mechanical vibration. The crystal formation results in release of the heat of fusion of water (80 cal/g water) causing the sample to warm to a point at which ice and solution exist in equilibrium, and the temperature remains constant for a period of time. Osmotic concentration, or osmolality, is expressed in units of milliOsmoles (mOsm) per kg of water, where one mOsm is equivalent to one mM of dissolved solute particles. A solution containing 1 Osmole (1000 mOsm) of dissolved solute per kg of water lowers the freezing point of water by 1.858°C. Therefore, the freezing point depression of the sample can be converted to units of osmolality, or osmotic concentration by dividing by 1.858. However, the osmometer does this calculation directly by converting the thermistor readings by direct comparison with readings obtained by using standard aqueous salt solutions of known osmolality. Measuring with an osmometer: 1. When you get to the osmometer, the machine should be on, the Operating Head should be down and there should be a clean, dry sample tube in the Refrigerator Well. Be sure that when you are done using the equipment, it is in the same condition in which you found it. 2. Be sure the osmometer is cooled: the cool light should be cycling on and off. 3. Raise the operating head by pushing the Head Release button. 4. The Range Switch should be in the 0-2 position and the Mode Switch in the RUN position. 5. Place 50 uL of sample into an osmometer tube using the pipet provided. Avoid capturing air in the bottom of the sample tube as this will adversely affect the reproducibility of your readings. 6. Place the sample tube into the Refrigerator Well. 90 7. Lower the Operating Head so that the Seed Wire and Probe enter the tube, and the Head latches in the down position, without forcing it. 8. The digital display will be reading negative numbers, which will start to become more positive, and will count up to almost 1000. When the number hits 1000, the sample is seeded with a high amplitude vibration of the seed wire (and the osmometer makes a loud, obnoxious noise). 9. After the sample is seeded, the numbers on the digital display will decrease until they read the osmolality of the solution. The READ light will come on and the head will pop up when the reading is complete. The number on the digital display is the osmolality of the solution. 10. Gently wipe off the Probe and Seed Wire with a clean kimwipe (DO NOT USE GAUZE PADS). Whenever the instrument is not in use, there should be an empty sample tube in the Refrigerator Well, to prevent frost buildup in the Well. When you are finished using the osmometer, you should turn it off and then lower the Operating Head into the empty tube to protect the Temperature Probe. Do not force a sample tube into a frosted Refrigerator Well. If the Well has accumulated frost, turn off the osmometer for several minutes to defrost, and remove the accumulated moisture from the Well with a “Q-tip”. 91 CHAPTER 14: USING MICROSCOPES 1. Treat a microscope gently. Each one costs about $1200. 2. Always use both hands when carrying a microscope. Place one hand under the base (bottom) of the microscope after lifting the microscope by its arm. 3. Do not move the lens down toward the slide to focus it; instead, run it down as far as it will go without touching the slide while watching from the side. Then move the lens up (turning the knob toward you) until it is in focus. 4. There are times when rule 3 does not strictly apply; the scanning lens cannot possibly go far enough down to touch the slide. In addition, you may want to make the fine adjustment with the low or high power lens in a downward direction. The important point is that you make a habit of never focusing down without being absolutely sure that it is safe. 5. Microscopes are good only if they are clean. Use only microscope lens cleaning paper to clean lenses, a single slight scratch can ruin the lens. NEVER wipe the lens using a circular motion; instead, tear a small piece of lens paper and sweep ONE TIME across the lens. Use a different piece of lens paper for each subsequent sweep, if necessary. 6. Whenever possible, hold the slide up to the light to identify the location of the specimen on it before placing it on the microscope stage. 7. You will save yourself a lot of time by always focusing under scanning or low power, then moving the higher power lenses into place. The microscopes are parfocal; that is, once the specimen is in brought into focus under one objective, it should remain in focus under all other objectives. To change from one objective to another, turn the nosepiece; do not push or pull on the individual objectives. 8. Keep the iris diaphragm closed as far as possible. Increasing the amount of light on the specimen decreases the depth of focus and the contrast. 9. To minimize eye fatigue, train yourself to keep both eyes open when looking through the ocular. 10. When observing living microorganisms, put a TINY drop of water containing the organism on the slide, followed by a tiny drop of slowing agent (“Protoslo”), if desired. To minimize air bubbles, hold the coverslip at an angle over the drop, with one edge touching the slide. Then lower the opposite edge of the coverslip to cover the drop. With larger microorganisms, like Paramecium, make a thin, uniform bead of Vaseline all the way around the edge of a plastic coverslip. Drop the coverslip on the slide, and slightly tap the top of it to make a good seal. 11. When drawing magnified specimens, always record the magnification at which the drawing was made. (The magnification of an object under the microscope is the product of the magnifications of the two lens systems, the objective and the ocular.) 92 12. When changing slides and when putting the microscope away, always position the scanning lens down. 13. If you have trouble seeing what you are looking for, ask the instructor for help. Ocular (10X) Nose Piece Objective lenses Arm Stage Iris Diaphragm Coarse Focus Fine Focus Light Source Power Switch/ Light Adjustment Knob Figure 14-1. A compound microscope. 93 CHAPTER 15: USING FIRST SEARCH AND OTHER LIBRARY RESOURCES This library information is provided to familiarize you with various resources available in the library and online that you will use when writing your Biology lab proposals and reports. Finding and citing literature in formal lab reports When preparing your formal lab reports, you will be expected to do the following: 1) Find at least four sources (journal or magazine articles, books, online articles) that are relevant to your Biology lab topic. Two of the sources must be from the primary literature (i.e. scholarly, peer-reviewed journals). These include: Original research articles: report original research Review articles: give an evaluation of primary literature Up to two of the sources may be from a book or edited book chapter Book by single or multiple authors: all information in the book is written by the author(s) on the book cover. Your textbook or lab manual can count as one of these sources, the other three must be non-131 course material sources. Edited book: contains a series of articles, each usually contributed by a different author. Each chapter is considered a different source citation. See the Student Survival Guide on how to cite books vs. chapters from edited books. 2) In your lab reports, show where you use information contained in these sources by a) correctly citing each in the text of the report. See "Citations within Text" in Chapter 6 of the Survival Guide. b) providing a complete, correctly formatted citation for each source in the Literature Cited section of your proposal or report. See "Literature Cited Page" in Chapter 6 of the Survival Guide. 3) attach a photocopy or printout of the first page of each cited source to the end of the report (do this for the first lab report only). A photocopy from your textbook or lab manual is not necessary. Remember, you must cite at least three sources in addition to your text or lab manual, and at least two of these must be primary sources (journal research or review articles). Finding and citing literature in lab proposals When preparing lab proposals, you will go through the research process outlined below to find at least two sources relevant to your lab topic. At least one must be an outside source. Provide appropriate citations in the text of your proposal, and full citations in the Literature Cited section. You do not need to include photocopies of your sources. Explore Your Topic • Your research will be easier if you understand what you are looking for. • If possible, write down your topic in the form of a question. • Write down the words that describe the main concepts of your topic. • For each concept within your topic, list as many synonyms and related words as possible. • For help finding these synonyms and understanding your topic, use sources that will give you background information, including: Biology textbooks Specialized dictionaries and encyclopedias, such as: 94 Dictionary of Microbiology and Molecular Biology McGraw-Hill Dictionary of the Life Sciences McGraw-Hill Encyclopedia of Science and Technology Search for Information - Books • • Use GABRIEL, the Library Catalog: http://gabriel.sewanee.edu Do a TITLE search for the books listed above. They will be found in the Reference area of the Library. Near these books will be others relating to Biology that will be useful. Often these books will lead you to other books, look for their references. • Do a WORD search for books dealing with your topic using some of the words you wrote down on your “Explore Your Topic” worksheet. Some of these should be in the General Collection of the Library. Near these books will be other related Biology books. Search for Information - Articles • Use a Biology Index. The most useful indexes are listed on the Biology Research Guide on the Library web page: http://library.sewanee.edu Two important indexes are: BasicBIOSIS, BioAgIndex (Biological and Agricutural Index). • Do a Keyword search using at least two of the concepts that you wrote down on your “Explore Your Topic” worksheet. If you get too many results, add a concept to narrow down your search. If you get too few results, take out a concept or change the wording to expand your search. • Choose several useful articles from your results. Locate the Journal and Magazine Articles in the Library • Use GABRIEL, the Library Catalog. • Do a TITLE search for the title of the journal (not the title of the article). • Also, check the list of Electronic Journals from the Library web page. If the Library owns the journal: Note the years we own to make sure we include the year of your article. Note the call number and go to the shelves to find the journal. If the Library does not own the journal: Order your journal article via Interlibrary Loan (forms available at the Reference Desk or from the Library web page) -- this process can take some time, two weeks or more. Choose another article to locate in the Library. Get Help Along the Way • Reference Librarians are in the Library to help you with your research. • Call them at 598-1368 • Email them askref@sewanee.edu Other Helpful Sources • • A Short Guide to Writing About Biology Scientific Style and Format: the CBE Manual for Authors, Editors, and Publishers