By GAINING or LOSING electrons
advertisement
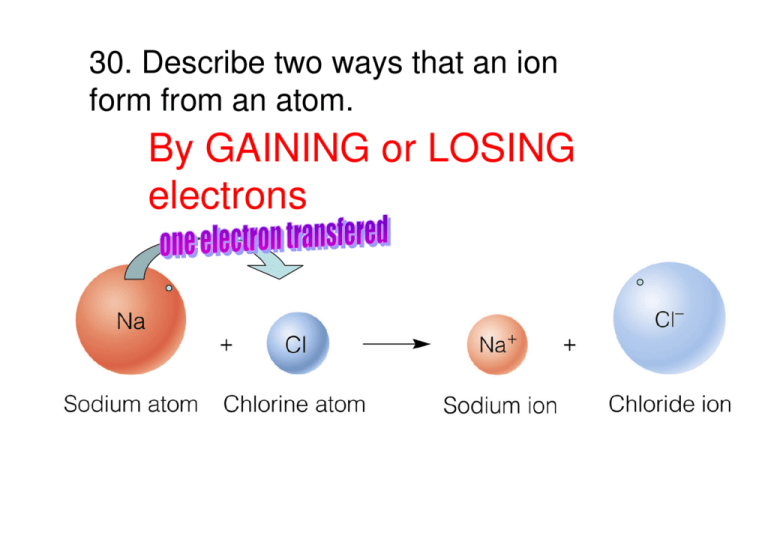
30. Describe two ways that an ion form from an atom. By GAINING or LOSING electrons #32 Name each ion in problem 31. Identify each as an anion or cation. a. Br -1 bromide, anion b. Na +1 sodium, cation c. As -3 arsenide, anion d. Ca +2 calcium, cation e. Cu +1 copper, cation f. H -1 hydride, anion #34 How many electrons does each atom have? What group is each in? a. nitrogen; 7 electrons group 5A b. lithium; 3 electrons group 1A c. phosphorus; 15 electrons group 5A d. barium; 56 electrons group 2A e. bromine; 35 electrons group 7A f. carbon; 6 electrons group 4A #36 How many electrons must each atom lose to attain a noble gas electron configuration? a. Ca needs to lose 2 electrons b. Al needs to lose 3 electrons c. Li needs to lose 1 electrons d. Ba needs to lose 2 electrons #38 Why do nonmetals tend to form anions when they react to form compounds? They are looking to fill their outer valence shell with 8 electrons http://jchemed.chem.wisc.edu/JCESoft/CCA/CCA0/Movies/NACL1.html #40 How many electrons must be gained by each of the following atoms to achieve a stable electron configuration? a. N gain 3 b. S gain 2 c. Cl gain 1 d. P gain 3 #42 Identify the kinds of ions that form each ionic compound. a. calcium fluoride, CaF2 Ca+2 F-1 b. aluminum bromide, AlBr3 Al+3 Br -1 c. lithium oxide, Li2O Li+1 O -2 d. aluminum sulfide, Al2S3 e. potassium nitride, K3N Al+3 K+1 S-2 N-3 #44 Which of the following pairs of elements will not form ionic compounds? a. sulfur and oxygen b. sodium and calcium c. sodium and sulfur d. oxygen and chlorine #46 Most ionic substances are brittle. Why? When hit the cations get close to the cations and the anions to the anions causing REPULSIVE FORCES #48 Explain briefly why metals are good conductors of electricity The loose highenergy valence electrons allow movement of electrons See World of Chemistry “Metals” minute 7:40 for model of “sea of electrons” #50 Name some alloys that you have seen or used today. See World of Chemistry “Metals” 16:50 for examples of alloys




