Equilibrium Mathematics
advertisement
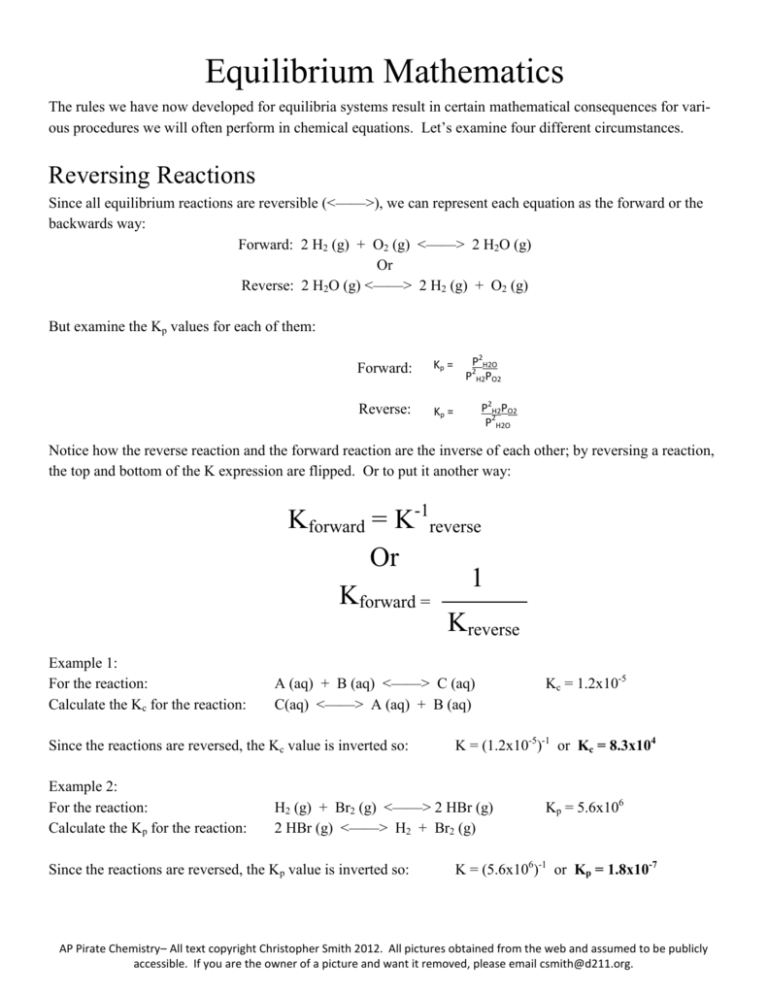
Equilibrium Mathematics The rules we have now developed for equilibria systems result in certain mathematical consequences for various procedures we will often perform in chemical equations. Let’s examine four different circumstances. Reversing Reactions Since all equilibrium reactions are reversible (<——>), we can represent each equation as the forward or the backwards way: Forward: 2 H2 (g) + O2 (g) <——> 2 H2O (g) Or Reverse: 2 H2O (g) <——> 2 H2 (g) + O2 (g) But examine the Kp values for each of them: Forward: Kp = Reverse: Kp = P2H2O 2 P H2PO2 P2H2PO2 P2H2O Notice how the reverse reaction and the forward reaction are the inverse of each other; by reversing a reaction, the top and bottom of the K expression are flipped. Or to put it another way: Kforward = K-1reverse Or 1 Kforward = Kreverse Example 1: For the reaction: Calculate the Kc for the reaction: A (aq) + B (aq) <——> C (aq) C(aq) <——> A (aq) + B (aq) Since the reactions are reversed, the Kc value is inverted so: Example 2: For the reaction: Calculate the Kp for the reaction: K = (1.2x10-5)-1 or Kc = 8.3x104 H2 (g) + Br2 (g) <——> 2 HBr (g) 2 HBr (g) <——> H2 + Br2 (g) Since the reactions are reversed, the Kp value is inverted so: Kc = 1.2x10-5 Kp = 5.6x106 K = (5.6x106)-1 or Kp = 1.8x10-7 AP Pirate Chemistry– All text copyright Christopher Smith 2012. All pictures obtained from the web and assumed to be publicly accessible. If you are the owner of a picture and want it removed, please email csmith@d211.org. Changing Stoichiometric Coefficients In some cases, we may want to change the stoichiometry of an equation to suit our needs. For example: Equation 1: H2O (g) <——> H2 (g) + 1/2 O2 (g) Kp = PH2P0.5O2 PH2O But what if instead of having the O2 be a coefficient of 1/2, we want whole numbers instead, so we can double the coefficients of all species to get: Equation 2: 2 H2O (g) <——> 2 H2 (g) + O2 (g) Kp = P2H2PO2 P2H2O Notice what happened to the Kp value? Each species was raised to the 2nd power because we doubled each of the coefficients. Consequently, Knew = Kxold x = ratio of stoichiometry Or to put it another way, if you double a reaction, every species in the K equation gets squared (x 2). If you triple a reaction, every species in the equation gets cubed (x3). If you cut an equation in half, every species get square-rooted (x1/2). Example 1: For the reaction: What is the Kc value for: 1/2 A (aq) + 1/2 B (aq) <——> C (aq) A (aq) + B (aq) <——> 2 C (aq) Since you are doubling the equation, the Kc value is squared or Example 2: For the reaction: What is the Kp value for: Kc = 4.3x10-2 Kc = ??? Kc = (4.3x10-2)2 = 1.85x10-3 P4 (s) + 6 Cl2 (g) <——> 4 PCl3 (g) 1/4 P4 (s) + 3/2 Cl2 (g) <——> PCl3 (g) Kp = 6.2x105 Kp = ???? Since you are cutting each species by 1/4, the Kp value is raised to the 1/4 power or Kp = (6.2x105)0.25 = 28.1 AP Pirate Chemistry– All text copyright Christopher Smith 2012. All pictures obtained from the web and assumed to be publicly accessible. If you are the owner of a picture and want it removed, please email csmith@d211.org. Adding Equations Together Often, you can use more than one equation added together to obtain another overall equation. What happens to the K values when multiple equations are added together? Let’s look at an example: Equation 1: S (s) + O2 (g) <——> SO2 (g) PSO2 PO2 Kp1= Equation 2: SO2 (g) + 1/2 O2 (g) <——> SO3 (g) Kp2= PSO3 PSO2 P0.5O2 Now if we add these two equations together we get the following: Equation 3: S (s) + SO2 (g) + 3/2 O2 (g) <——> SO2 (g) + SO3 (g) But we should cancel the SO2 (g) because they appear on both sides so: Equation 3: S (s) + 3/2 O2 (g) <——> SO3 (g) Kp = PSO3 P1.5O2 How do the Kp values of equation 1 and 2 relate to equation 3? If you multiply Kp1 and Kp2 together, you get Kp3: Kp1 x Kp2 = Kp3 PSO2 PO2 x PSO3 PSO2P0.5O2 = Kp3 PSO2 PO2 x PSO3 PSO2P0.5O2 = Kp3 PSO3 PO2P0.5O2 = Kp3 PSO3 P1.5O2 = Kp3 Which matches the value we expected from above Ktot = K1 x K2 x K3…. AP Pirate Chemistry– All text copyright Christopher Smith 2012. All pictures obtained from the web and assumed to be publicly accessible. If you are the owner of a picture and want it removed, please email csmith@d211.org. Example 1: Equation 1: H2C2O4 (aq) + H2O (l) <——> HC2O4-1 (aq) + H3O+1 (aq) K1 = 2.3x10-2 Equation 2: HC2O4-1 (aq) + H2O (l) <——> C2O4-2 (aq) + H3O+1 (aq) K2 = 4.7x10-5 What is the K value for the equation: Equation 3: H2C2O4 (aq) + 2 H2O (l) <——> C2O4-2 (aq) + 2 H3O+1 (aq) K3= ???? To get equation 3, you have to add together equations 1 and 2, combine like terms, and cancel: H2C2O4 (aq) + H2O (l) + HC2O4-1 (aq) + H2O (l) <——> HC2O4-1 (aq) + H3O+1 (aq) + C2O4-2 (aq) + H3O+1 (aq) H2C2O4 (aq) + 2 H2O (l) <——> C2O4-2 (aq) + 2 H3O+1 (aq) So K3 = K1 x K2 = (2.3x10-2)(4.7x10-5) = 1.08x10-6 Example 2: Equation 1: C (s) + CO2 (g) <——> 2 CO (g) Kp = 2x1013 Equation 2: CO (g) + Cl2 (g) <——> COCl2 (g) Kp = 4x10-4 What is the K value for the equation: Equation 3: C (s) + CO2 (g) + 2 Cl2 (g) <——> 2 COCl2 (g) To get equation #3 you have to leave equation #1 the same but you have to double equation #2 and then add them together: Equation 1: C (s) + CO2 (g) <——> 2 CO (g) Kp = 2x1013 Equation 2: 2 CO (g) + 2 Cl2 (g) <——> 2 COCl2 (g) Kp = (4x10-4)2 = 1.6x10-7 Remember that when you double an equation you have to square (x2) the K value Then add them together and cancel terms: Equation 3: C (s) + CO2 (g) + 2 CO (g) + 2 Cl2 (g) <——> 2 CO (g) + 2 COCl2 (g) So K3 = K1 x K2 = (2x1013)(1.6x10-7) = 3.2x106 AP Pirate Chemistry– All text copyright Christopher Smith 2012. All pictures obtained from the web and assumed to be publicly accessible. If you are the owner of a picture and want it removed, please email csmith@d211.org. Kc vs Kp We have seen problems where we have solved for Kc and other problems where we have used Kp. Are Kc and Kp interchangeable? Are they the same? If not, is there ever a circumstance where they are the same? Let’s examine a simple equilibrium example: CaCO3 (s) <——> CaO (s) + CO2 (g) Write Kc for this reaction: Write Kp for this reaction: Kc = [CO2] Kp = PCO2 Does Kc = Kp? Does [CO2] = PCO2 ? Clearly not. Pressure does not equal concentration. But can we turn pressure into concentration? Can we equate them? We can if we remember the ideal gas law: PV = nRT We see that there is pressure (P) but where is concentration? Remember that concentration is moles per liter so: P = (n/V)RT or substituting in [X] for (n/V) we get: P = [X]RT So pressure does not equal concentration but if we multiply the concentration by RT, then we get pressure: Moles * Liter atm * K Liter mole K [X] R T So every time you change a concentration [X] into a pressure P, you have to multiply the concentration by RT. This is going to result in some strange math, however. Examine the following example AP Pirate Chemistry– All text copyright Christopher Smith 2012. All pictures obtained from the web and assumed to be publicly accessible. If you are the owner of a picture and want it removed, please email csmith@d211.org. Example 1: The Kc value is 4.2x10-4 for the reaction: PCl5 (g) <——> PCl3 (g) + Cl2 (g) Calculate Kp at 273 K. Kc has the equation Kc = [PCl3][Cl2] [PCl5] Since we need to convert each of those concentration values into pressure, we need to multiply each by RT: But look what happens. We have (RT)2 on top and RT on the bottom so one of those RT values cancels Kp = ([PCl3]RT)([Cl2]RT) [PCl5]RT Which leaves us with Kc = But since Kp = ([PCl3]RT)([Cl2]RT) [PCl5]RT Kp = ([PCl3])([Cl2])RT) [PCl5] [PCl3][Cl2] [PCl5] This equation becomes: Kp = Kc(RT) Consequently: Kp = 4.2x10-4 (0.0821 Latm/molK)(273 K) = 9.41x10-3 But that sure seemed like a lot of work, right? Is there a shortcut we could use so we don’t have to do that long derivation anymore? There is if we look at the big picture of things. Let’s examine a complex example: Example 2: The Kc value is 8.1x10-2 for the equation: 2 NH3 (g) <——> N2 (g) + 3 H2 (g) Calculate Kp at 300 K. Doing the same thing as last time we get that: Kc = [N2][H2]3 Or to put it another way: Kc = [N2][H2][H2][H2] 2 [NH3] [NH3][NH3] Since each [x] needs to be multiplied by RT we get: Or: Kc = [N2][H2]3 (RT)4 [NH3]2 (RT)2 Kc = [N2][H2]3 (RT)2 Kc = [N2](RT)[H2](RT)[H2](RT)[H2](RT) [NH3](RT)[NH3](RT) [NH3]2 Notice how there is (RT)2 on bottom and (RT)4 on the top? Examine the equation. Notice how there are two moles of gases on the reactant side and 4 moles of gases on the product side? 2 NH3 (g) <——> N2 (g) + 3 H2 (g) Since each gas gets modified by RT we can streamline the process by recognizing that every 1 mole of gas on each side of the equation will cancel out. Only if a side has more gas than the other will RT matter. AP Pirate Chemistry– All text copyright Christopher Smith 2012. All pictures obtained from the web and assumed to be publicly accessible. If you are the owner of a picture and want it removed, please email csmith@d211.org. Thus, what matters is the change in the number of moles of gas or to put it another way: Kp = Kc (RT)gas Now to finish this example: The Kc value is 8.1x10-2 for the equation: 2 NH3 (g) <——> N2 (g) + 3 H2 (g) Calculate Kp at 300 K. Since there are 2 moles of gas on the left and 4 on the right, the gas = (prod)-(react) = 4-2 = +2 so Kp = Kc (RT)+2 Kp = (8.1x10-2)[(0.0821)(300)]+2 Kp = 49.1 Example 3: The Kc value is 2.3x104 for the equation: 2 SO3 (s) <——> 2 SO2 (g) + O2 (g) Calculate Kp at 500 K Kp = Kc(RT)gas Since there are 3 moles of gases on the product side and 2 moles of gases on the reactant side: gas = 3-2 = +1 So 4 Kp = (2.3x10 )[(0.0821)(500 K)]+1 = 9.4x105 Example 4: The Kc value is 1.8x10-6 for the equation: Ti (s) + 2 Cl2 (g) <——> TiCl4 (l) Calculate Kp at 750 K Kp = Kc(RT)gas Since there are 0 moles of gases on the product side and 2 moles of gases on the reactant side: gas = 0-2 = -2 So -6 Kp = (1.8x10 )[(0.0821)(750 K)]-2 = 4.75x10-10 Example 5: The Kp value is 6.7x10-7 for the equation: 3 O2 (g) <——> 2 O3 (g) Calculate Kc at 250 K Kp = Kc(RT)gas Since there are 2 moles of gases on the product side and 3 moles of gases on the reactant side: gas = 2-3 = -1 So -7 6.7x10 = Kc[(0.0821)(250 K)]-1 = 1.4x10-5 AP Pirate Chemistry– All text copyright Christopher Smith 2012. All pictures obtained from the web and assumed to be publicly accessible. If you are the owner of a picture and want it removed, please email csmith@d211.org. So is there ever a circumstance where Kp = Kc ? Yes, if the number of moles of gas on each side are equal. Examine: H2 (g) + Cl2 (g) <——> 2 HCl (g) Kp = Kc(RT)gas Since there are 2 moles of gases on the product side and 2 moles gas on the reactant side gas = 2-2 = 0 So Kp = Kc [RT]0 Since anything raised to the 0 power = 1 we get: Kp= Kc So: If there are the same moles of gas on each side of the equation: Kp = Kc Summary Kforward = K-1reverse Knew = Kxold where x = ratio of stoichiometry (doubled, tripled, halved, etc.) Ktotal = K1x K2 x K3 … When adding equations together Kp = Kc (RT)gas Where gas is the change in the number of moles of gases (products-reactants) Kp = Kc If the number of moles of gases on each side of the equation is the same AP Pirate Chemistry– All text copyright Christopher Smith 2012. All pictures obtained from the web and assumed to be publicly accessible. If you are the owner of a picture and want it removed, please email csmith@d211.org.