International Journal of Pediatric Otorhinolaryngology 76 (2012) 311–318
Contents lists available at SciVerse ScienceDirect
International Journal of Pediatric Otorhinolaryngology
journal homepage: www.elsevier.com/locate/ijporl
Review Article
Otolaryngological aspects of sudden infant death syndrome
Tal Marom a,*, Udi Cinamon a, Paul F. Castellanos b, Marta C. Cohen c
a
Department of Otolaryngology – Head & Neck Surgery, Edith Wolfson Medical Center, Tel Aviv University Sackler School of Medicine, Holon, Israel
Division of Otolaryngology – Head and Neck Surgery, University of Alabama at Birmingham, 1530 3rd Avenue South, Birmingham, AL 35294, United States
c
Histopathology Department, Sheffield Children’s NHS Foundation Trust, Western Bank, Sheffield, Yorkshire S10 2TH, United Kingdom
b
A R T I C L E I N F O
A B S T R A C T
Article history:
Received 25 October 2011
Received in revised form 8 December 2011
Accepted 9 December 2011
Available online 11 January 2012
Introduction: Sudden infant death syndrome (SIDS) is characterized by the sudden death of an apparently
otherwise healthy infant, typically during sleep, and with no obvious case after a thorough post-mortem
and scene death examination.
Objective: To address the problem from the otolaryngologist’s perspective, describe relevant pathologies,
discuss controversies and suggest preventive measures in high-risk populations.
Methodology: A MEDLINE search and hand search were conducted to identify reports published between
1969 and 2011 in the English language on the pathophysiology of SIDS related to the head and neck
organs. Search terms included SIDS (MeSH term), SIDS and pathophysiology (text words), and SIDS and
autopsy (text words).
Discussion: A growing number of reports suggested head and neck organs involvement in SIDS autopsies.
Laryngeal, oropharyngeal, maxillofacial, otologic, cervical vascular abnormalities and infectious
etiologies, were recognized and discussed.
Conclusions: Otolaryngologists should be aware of relevant pathologies, as some are treatable, if
identified early enough in infancy. A proactive risk-management approach is warranted in infants
presenting with certain abnormalities reviewed here.
ß 2011 Elsevier Ireland Ltd. All rights reserved.
Keywords:
Sudden infant death syndrome
Hypoxemia
Airway
Obstruction
Head and neck
Contents
1.
2.
3.
4.
5.
6.
Introduction . . . . . . . . . . . . . . . . . . . . . . .
Objective . . . . . . . . . . . . . . . . . . . . . . . . .
Methodology . . . . . . . . . . . . . . . . . . . . . .
Results . . . . . . . . . . . . . . . . . . . . . . . . . . .
Laryngeal skeleton changes . . . . .
4.1.
Upper respiratory tract infections
4.2.
Laryngopharyngeal reflux . . . . . . .
4.3.
Phonation . . . . . . . . . . . . . . . . . . .
4.4.
Oropharyngeal pathologies. . . . . .
4.5.
Acute otitis media. . . . . . . . . . . . .
4.6.
Inner ear malfunction . . . . . . . . . .
4.7.
Sleep apnea and sleep position . .
4.8.
Maxillofacial deformities . . . . . . .
4.9.
4.10. Carotid body abnormalities . . . . .
4.11. Aberrant cervical blood vessels . .
Discussion . . . . . . . . . . . . . . . . . . . . . . . .
Conclusions . . . . . . . . . . . . . . . . . . . . . . .
References . . . . . . . . . . . . . . . . . . . . . . . .
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
* Corresponding author at: Department of Otolaryngology – Head & Neck
Surgery, Edith Wolfson Medical Center, P.O. Box 5, 58100 Holon, Israel.
Tel.: +972 3 5028651; fax: +972 3 5028199.
E-mail address: maromtal@orange.net.il (T. Marom).
0165-5876/$ – see front matter ß 2011 Elsevier Ireland Ltd. All rights reserved.
doi:10.1016/j.ijporl.2011.12.008
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
311
312
312
312
312
312
313
314
314
315
315
315
315
316
316
316
317
317
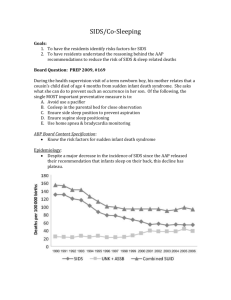
1. Introduction
Sudden infant death syndrome (SIDS), as defined by the
National Institute of Child Health and Human Development [1],
312
T. Marom et al. / International Journal of Pediatric Otorhinolaryngology 76 (2012) 311–318
is the sudden death of an infant under 1 year of age, which remains
unexplained after a thorough case investigation, including
performance of a complete autopsy, examination of the death
scene, and review of the clinical history.
To date, SIDS is still the most frequent cause of death for infants
in this age group in most industrialized countries. Peak mortality is
from the 2nd to the 4th month [2]. A higher mortality rate is
reported among male infants (60%), and during the cold season
(75%), when infectious diseases are more likely to occur [2].
Many risk factors for SIDS have been investigated throughout
the years, which have been categorized as either intrinsic or
extrinsic. Examples for intrinsic risks include: male sex; an
insertion/deletion polymorphism in the serotonin transporter
protein gene expressed in the arcuate nucleus (a hypothalamic
nucleus which has a proven role in controlling respiratory
frequency), nucleus raphé obscurus (a medullary nucleus which
controls expiration), and other medullary regions; belonging to a
Black or Native American ethnic group of origin; prematurity;
perinatal exposure to smoking; parental smoking, ethanol and
drug abuse. Extrinsic risk factors include sleeping on the side; soft
bedding; low socioeconomic status; bed sharing and concurrent
infections [3]. A recent report suggested that many SIDS victims
shared multiple risk factors. In that series, most of SIDS cases had
more than 1 risk, whereas risk-free cases were rare [4].
Several mechanisms have been proposed for SIDS: abrupt
airway obstruction while sleeping and upon arousal, re-breathing
of expired gases resulting in hypercarbia and hypoxic coma;
thermal stress; undiagnosed upper airway infection which was
critical enough to effect respiratory functions; fatal unexpected
apnea; cardiac arrhythmia, such as Brugada-type ECG and long QT
intervals, and poisoning by either immunizations or other toxic
gases [5,6]. This spectrum of theoretical mechanisms implicates
the interaction of multiple factors in the pathogenesis of SIDS. The
‘‘Triple-Risk Model’’, presented in 1994, suggested that SIDS may
occur once three factors presented simultaneously: an underlying
vulnerability in the infant, a critical developmental period, and an
exogenous stressor [7].
SIDS is a sub-category of a larger sudden unexpected death in
infancy (SUDI) group cases, which refers to any death that presents
suddenly and unexpectedly in an infant. While in approximately
20% of SUID cases a cause of death is found, i.e., infection,
aspiration, domestic violence (suffocation) and other causes, the
rest large majority of sudden death in infancy will remain
unexplained, therefore categorized as SIDS [8].
2. Objective
Although the specific cause of death remains obscure in most
SIDS cases, there is growing body of evidence from autopsies, which
suggests head and neck pathology is involved in some SIDS cases.
The purpose of this review is to present the problem from the
otolaryngologist’s perspectives, describe relevant pathologies,
discuss controversies and suggest a proactive approach in subsets
of infants with certain abnormalities which put them at risk for SIDS.
years 1969–2011 and English, respectively. As SIDS definition
changed throughout time, we excluded reports which were not
consistent with SIDS definition at the time of their publishment.
4. Results
4.1. Laryngeal skeleton changes
The larynx undergoes significant critical developmental
changes in the first year of life. Infants have a proportionately
larger tongue situated within the oropharynx blocking the entire
aperture except when crying; they are, therefore, obligate nose
breathers. They also have narrower nostrils in relation to the
trachea, a higher and smaller larynx, and an elongated more rigid
omega-shaped epiglottis. There is functionally no clear distinction
between the epiglottis and the soft palate as they abut each other
and function as a single unit. Consequently, the lateral borders of
the epiglottis are pushed against the posterior pharyngeal wall.
This position allows the omega-shaped epiglottis to interlock with
the soft palate when breast-feeding. This barrier creates a straight
route for air to travel from nose to lungs while breastfeeding, thus
allowing the infant to breathe and swallow simultaneously [9]. The
upper airways of normal infants are smaller in both inspiration and
expiration at 6 weeks of age, when compared to the neonatal
period, due to thickening of the mucous membrane lining, or in
some cases, due to adenoidal growth, perhaps related to bacterial
or viral infections.
The age of 4–6 months is considered a cardinal transitional
period from obligate nasal breathing to oral respiration. The
posterior aspect of the tongue gradually slides down and forms the
new anterior border of the oropharynx, due to its relative large size
within the oral cavity [10]. The larynx and epiglottis descend away
from the soft palate down in the neck to create a common passage
for air, food, and liquid (Fig. 1).
This shift reflects a period of potential respiratory instability,
when the laryngeal inlet is exposed to both food and fluids during
breathing and swallowing. Maturation of vagus-mediated reflexes
in the growing larynx protects the airway from aspirations. Any
developmental failure of the laryngeal framework anatomy may
jeopardize airway protection. An undescended larynx may narrow
the upper airway at the supraglottic level, in addition to the natural
relative stenosis at the subglottic and cricoid level. Therefore,
aspiration at this unique period of maturation may be significant.
Some studies have argued against this hypothesis. Stephens
et al. [11] measured the uvulo-epiglottis and sella turcicaepiglottis distances in MR scans and plain lateral neck radiographs
in infants aged 1–357 days. They failed to demonstrate the change
in the rate of laryngeal descent between the ages of 2–4 months,
which is the peak age of SIDS. Given that the main guarding
mechanism of the infant airway is the epiglottis, even in infants
with laryngomalacia, its function as the laryngeal barrier is usually
maintained [12]. Moreover, most laryngeal findings in autopsies
are unremarkable.
4.2. Upper respiratory tract infections
3. Methodology
We performed a computer literature search in the MEDLINE
electronic database to identify studies that answered the question
of interest. For this purpose, we used the following free-text terms:
‘‘Sudden infant death syndrome’’ with ‘‘pathophysiology’’ or
‘‘autopsy/post-mortem or ‘‘larynx’’ or ‘‘carotid’’ or ‘‘maxilla’’ or
‘‘airway’’ or ‘‘reflux’’ or ‘‘pharynx’’ or ‘‘anomalies’’ or ‘‘head and
neck’’ or ‘‘ear’’, and limited to ‘‘human.’’ In addition, extensive
hand-searching of the references of all relevant studies was also
performed. Time and language limitations were applied to the
Several publications have suggested that a significant number of
currently unexplained SIDS deaths may be mediated through
abnormal systemic immune responses to otherwise transient or
subclinical infections, especially in the upper airway, implying that
the spectrum of potential mechanisms of infection-related deaths in
SIDS may be wider than simply a consequence of direct tissue
invasion and destruction (Fig. 2). Post-mortem cultures vary widely
and depend on the interpretation of results and methods of
specimen collection. In theory, pro-inflammatory cytokines induced
by infections can cause respiratory and cardiac dysfunction, pyrexia,
T. Marom et al. / International Journal of Pediatric Otorhinolaryngology 76 (2012) 311–318
313
Fig. 1. The interlocked soft palate and epiglottis in infants due to the elevation of the larynx allows simultaneous breathing and drinking (left). The position of the infant’s
tongue entirely within the oral cavity allows the distinctly omega-shaped epiglottis to interlock with the soft palate when feeding (right). Milk flows through the lateral
faucium channels.
Reproduced and adapted with permission from: Pediatric airway management, in: B.T. Finucane, B.C. Tsui, A.H. Santora, (Eds.), Principles of Airway Management, 4th ed.,
Springer, NY, 2011.
shock, hypoglycemia and diminished arousal. At the age of 2–4
months, most infants have already lost their maternal antibodies,
and become carriers of both Streptococcus pneumoniae in the
nasopharynx and Staphylococcus aureus in the anterior nares. Both
have the potential to cause substantial infections at this age group. In
addition, the prone position can raise the core body temperature and
theoretically increase replication of bacteria, turning commensal
organisms into pathogens [13].
One of the largest autopsy studies reported that in 57/116 (49%)
of SIDS cases, a potentially pathogenic organism was isolated from
at least one site, suggesting that infection may indeed be an
important contributory factor in SIDS [14]. Most of the isolated
pathogens were commensals of the upper respiratory tract, and
included Streptococcus pneumoniae, Haemphilus species, Staphylococcus aureus and group B Streptococcus as the most common
species. The authors concluded these pathogenic species contribute to SIDS in the inflammatory/infectious pathway.
Human parainfluenza virus (HPIV) was associated with SIDS.
This virus commonly causes upper airway infections and croup in
children, due do its high affinity to the larynx and trachea. In most
cases, recovery is likely with minimal medical therapy. However,
laryngeal edema resulting in airway compromise may occur. An
autopsy of a SIDS victim revealed a predominantly lymphocytic
infiltrate within the laryngotracheal mucosa, which was consistent
with infection caused by HPIV that was cultured from the trachea
at autopsy. The authors suggested HPIV-induced laryngospasm as
the cause of death [15]. It has been demonstrated that sleeping in a
prone position while having an upper respiratory tract infection
was associated with significantly increased bacterial counts,
including increased colonization by staphylococci [16]. How this
observation may lead to SIDS is still unknown.
Upper respiratory viral infections intensify laryngeal reflex
responses in animal models. It has been shown that cytokines
produced in the laryngeal mucosa during respiratory syncytial
viral (RSV) infection are transported retro-axonally to brain stem
centers that potentially regulate swallowing and respiratory
pattern [17]. Noteworthy is that interleukins are elevated in the
cerebrospinal fluid of many SIDS infants compared with controls.
In the same report, interleukins were found to be elevated in the
brain stem of SIDS infants [18].
Infections are very common in the first year of life; thus, if
infection plays a role in the cause of SIDS, a biological risk or
predisposing factor may be involved. This has led to several
investigations, which identified IL-1 and its receptor as the key
ligands involved in Staphylococcus aureus-induced septic shock in
several SIDS victims [19]. The origins of these fatal infections were
in the upper respiratory tract.
4.3. Laryngopharyngeal reflux
Fig. 2. Tracheal mucosal inflammation (arrow) in a SIDS victim (H&E, 10).
Extra-esophageal gastric reflux can be a major cause of
cardiorespiratory events in early postnatal life, especially via the
triggering of fetal-type laryngeal chemoreflexes (Fig. 4). It is very
common in young infants, and it is a recognized cause of ALTE.
Moreover, gastric contents are found in the upper airway system
and the lungs of many SIDS victims. It is presumed that aspiration
314
T. Marom et al. / International Journal of Pediatric Otorhinolaryngology 76 (2012) 311–318
Fig. 3. Aspiration of food in bronchial lumen (arrow) in a SIDS victim. The fresh
hemorrhage in the surrounding alveoli indicates a vital lesion (H&E, 20).
of these contents is an agonal preterminal event as a result of a
laryngopharyngeal reflux event (Fig. 3).
Additionally, laryngopharyngeal reflux can activate important
upper airway reflexes such as the laryngeal chemoreflexes (LCR),
whose vagal component can be responsible for significant
cardiorespiratory inhibition in certain circumstances [20]. Several
laryngeal receptors have been implicated as being responsible for
the LCR. It is generally accepted that laryngeal chemoreceptors,
which are densely present on the laryngeal surface of the
epiglottis, aryepiglottic folds and the cuneiform processes are
involved in the LCR [21]. Other mucosal receptors which contain
unmyelinated C fiber endings may also be involved. These are
stimulated when exposed to certain chemicals such as extracellular H+ ions [22]. Following activation, the sensory neural
information reaches the recurrent laryngeal nerve from the
superior laryngeal nerve and the Nerve of Galen, which may result
in laryngospasm.
When compared to fetal LCR, mature LCR stimulation primarily
results in short apnea, laryngeal closure, expiratory reflex, cough
and swallowing, as well as arousal if it occurs during sleep. While
postnatal maturation of the LCR has been described in newborn
mammals, current data suggest that LCR in the healthy, full-term
neonate do not include clinically significant cardiorespiratory
inhibition. In contrast, fetal-type LCR with apneas, bradycardias
and hemoglobin desaturations, which can at times be life
threatening, are observed in certain abnormal neonatal conditions, especially in premature newborns. LCR-related cardiorespiratory events are mostly observed in newborns and young
infants. Thach et al [23] proposed that gastroesophageal reflux
could cause SIDS. These authors challenged the common notion
that aspirated gastric contents, frequently found in lungs and
airways of SIDS victims, should be seen as resulting from the
agonal process and thus a non-SIDS specific process. They argued
that impairment of auto-resuscitation mechanisms provoked in
the normal infant by aspirated liquid, as demonstrated in an
animal model, may play a key role in the mechanisms leading to
death in several SIDS cases.
Laryngopharyngeal reflux-related LCR does not seem to cause
SIDS by itself, but rather represents as a trigger, which can initiate a
chain of events ultimately leading to death if the multiple recovery
mechanisms (arousal, anoxic gasping) fail. In a recent report, 4
cases of sudden infant death in which gastroesophageal reflux was
a contributory, if not a causative, factor were described. The
authors based their conclusion on histopathological studies
showing gastric contents in the lungs associated to features of a
lesion that had developed pre-mortem rather than been a postmortem artifact [24]. Yet, many uncertainties persist with regard
to the exact role of gastroesophageal reflux in relation to
cardiorespiratory events.
4.4. Phonation
Several studies have investigated cry patterns in infants and
their possible relations to SIDS. Recordings of a few very young
babies who eventually died later in infancy and were tagged as
SIDS concluded that the cry was inconclusive, as it was reported as
either short and high-pitched [25] or long and low-pitched [26].
Assuming SIDS infants and their siblings are more alike than
different, there is an expectation that similar crying behaviors
would be apparent (except for a lower intensity, as recorded in the
very few observations). An acoustic analysis research was designed
to explore patterns which may be relevant to laryngeal pathology.
When compared to healthy controls, analysis of cries in SIDS
siblings revealed substantial differences in acoustic parameters,
such as first spectral peak (the frequency value associated with the
first amplitude maximum across a crying episode) and spectral tilt
(a neurophysiological representation of how quickly amplitudes of
harmonics decline during cry) [27]. The results so far indicate a
high-energy cry in SIDS victims. Its mechanism and significance
are yet unknown.
4.5. Oropharyngeal pathologies
Fig. 4. Esophagitis with mild basal cell hyperplasia and intraepithelial eosinophils
(arrows), consistent with gastro-oesophageal reflux (H&E; 20).
Several reports of SIDS victims describe oropharyngeal structural anomalies. A SIDS case autopsy revealed an aberrant uvula
which descended to the level of the vocal cords. This might have
caused intermittent laryngospasm with subsequent symptoms of
cough and airway obstruction, ending in a fatal outcome [28].
Large thyroglossal cysts were reported in several SIDS autopsies,
some having a substantial lingual component. In these cases,
severe airway obstruction was presumed to be caused by a mass
effect by displacing the epiglottis posteriorly, causing an obstruction of the hypopharynx [29,30].
The position of the tonsils enables handling airborne and
alimentary antigens. Moreover, the palatine tonsils may play an
T. Marom et al. / International Journal of Pediatric Otorhinolaryngology 76 (2012) 311–318
important role in ‘‘priming’’ the bronchus- and gut-associated
lymphoid tissues. Data from the 1990s demonstrated that SIDS
infants have a stimulated immune system at time of death.
Whereas most studies have been on the secretory immune system,
the palatine tonsils were investigated separately for the presence
of immunoglobulins [31–33]. SIDS infants had statistically
significant higher concentrations of IgG and IgA-lymphocytes
compared to control infants. The authors concluded that the
stimulated immune system in the upper aerodigestive tract was
most likely cause being an infection [34]. Subsequent studies
demonstrated elevated circulating IgA levels in some ALTE and
‘near-miss’ SIDS infants, thus supporting the hypothesis of a
mucosal immune deregulation component [35]. However, this
theory has been partially abandoned throughout the years, as it
may not fully explain key processes leading to SIDS [36].
4.6. Acute otitis media
Acute otitis media (AOM) is one of the most common diseases of
early childhood. Epidemiological studies report the prevalence rate
of AOM to be 17–20% within the first 2 years of life [37]. Although
rare, serious intracranial complications of AOM, may lead to a fatal
outcome, therefore an unrecognized complicated AOM may
potentially be the cause of SIDS.
‘‘Silent’’ otitis media was coined by Paparella in 1980, and it
refers to a chronic pathological inflammation behind an intact
tympanic membrane, which may be clinically ‘‘undetected’’ or
‘‘undetectable’’ [38]. Though rare, infants reported with ‘‘silent’’
AOM had a relatively high rate of an otomeningitic complication
and a fatal outcome, as learned from a few temporal bone
histopathological studies obtained from SIDS victims [39,40].
Swab sampling from the middle ear has become a routine in
SIDS autopsy guidelines in a few institutions [13]. The presence of
an exudate in the middle ear was detected in 31/116 (27%) of SIDS
cases in a large UK cohort study published recently [13]. A number
of potential pathogens were found in these cases (48% of those
tested), which highlights the need for further assessment of a
potential role of middle ear infection as a cause or a contributory
factor in SIDS. In another case-series of 11 autopsies in unexpected
death in Japanese infants under the age of 1 year, there were 3
cases with AOM [41]. AOM, as such, was not a cause of death in
these cases. However, all infants with AOM had other risk factors
for SIDS: bottle-fed, CMV infection (2 cases) and tobacco smoke
exposure (3 cases) [42,43].
315
Additional research is under way to explore more fully the link
between inner ear malfunction and SIDS. In a recent experiment in
mice, intratympanic gentamicin induced inner ear hair cell
damage, which was validated with hearing and vestibular tests,
in addition to immunoflourescent microscopy. These mice
demonstrated a suppressed respiratory response to inhaled CO2
when compared to control mice with sham procedures. This data
suggest the integral role of the inner ear and its interconnecting
pathways in respiratory control, which may malfunction in the
SIDS scenario [45]. To date, there is no controlled study which
questioned this issue thoroughly.
4.8. Sleep apnea and sleep position
Traditionally, SIDS has been thought to occur during sleep. The
apnea hypothesis dominated the explanation for SIDS in the 1970s
and 1980s, following the report of sleep studies showing frequent
apneas in infants who had prolonged apnea and cyanotic episodes
during sleep. Two of the infants studied subsequently died, and
thus, these were labeled as SIDS. These two infants were siblings
and their three older siblings had all died as well.
These speculations were tested in several studies throughout
the years, which all failed to validate that sleep apnea is indeed the
sole etiology of SIDS. Cardiorespiratory recordings of infants dying
do not exhibit increased respiratory effort. Moreover, there are
reports of unexpected infant death with similar demographic and
pathological profiles to SIDS, except for the death occurred while
being awake, either while being fed or held in caregiver’s arms
[46].
Following large population studies in the 1980s and 1990s, it
was believed that prone sleeping position was causally associated
with SIDS. This has led to large ‘‘back to sleep’’ campaigns, which
recommended that infants should be placed to sleep on their
backs. Factors that might trigger infant death in the prone
position include asphyxia due to airway compression or
rebreathing of exhaled gases in the face-down position [47];
impaired heat loss with subsequent hyperthermia when the face
is pressed against bedding [48]; impaired cardiorespiratory
regulation related to heat stress; and compromised arousal in
response to asphyxia were extensively studies which yielded
conflicting results [49]. All of these possible etiologies were
extensively studied yielded conflicting results [50,51]. If not bad
enough for this explanation for SIDS, about 10% of SIDS cases
occurred in infants sleeping in the supine position, without any
other apparent contributing factor.
4.7. Inner ear malfunction
4.9. Maxillofacial deformities
The inner ear vestibular apparatus has been demonstrated to
play an important role in respiratory control during sleep. Thus, a
perinatal inner ear insult resulting in the disruption of vestibular
function may play a critical role in the predisposition to SIDS.
Newborn hearing screening using transient-evoked otoacoustic
emissions (TE-OAE) is now the standard practice in many
countries. The results in SIDS victims were compared to matched
controls [44]. TE-OAE screening results of SIDS infants demonstrated significantly decreased signal-to-noise ratios at 2000, 3000,
and 4000 Hz on the right side, when compared to healthy control
infants. That unilateral difference in cochlear function was
proposed to help identify infants at risk of SIDS during the early
postnatal period, with a simple non-invasive hearing screen. The
proported pathophysiology is an injury to the inner hair cells,
which facilitate transmission of blood carbon dioxide levels to the
brain. This causes disruption of respiratory control during sleep,
predisposing the infant to SIDS. However, failure in TE-OAE may be
attributed to other causes, such as middle ear effusion. Thus, which
is the cause and which is the effect is still uncertain.
The infant’s jaw at birth is almost horizontal and the
articulation with the skull is unstable. The temporo-mandibular
ligament, a thickening of the joint capsule which extends from the
lateral surface of the head of the mandible to the temporozygomatic ramus, is relatively flexible. The mandible can be easily
displaced posteriorly. A hypermobile mandible may augment
airway obstruction occurring at the level of the posterior pharynx
when in the prone sleeping position. It has been proposed that
airway obstruction occurring at the level of the posterior pharynx
due to muscle relaxation during REM sleep might lead to
subsequent hypoxia, cardiac arrest and death [47,52]. Petechial
hemorrhages in the pleura and intrathoracic part of the thymus,
demonstrated in many SIDS autopsies, might be indicative of the
increased negative intrathoracic pressure prior to death, suggesting an increased respiratory effort against an obstruction [53]. The
posterior position of the maxilla and mandible narrowing the
retropalatal airway were observed in lateral cephalograms taken at
necropsy of SIDS infants. This was considered a predisposing factor
316
T. Marom et al. / International Journal of Pediatric Otorhinolaryngology 76 (2012) 311–318
to SIDS, in a small UK study which consisted 15 cases [54].
Nevertheless, it is complicated to validate this postulated
mechanism of maxillomandibular misalignment. Other nonspecific facial dysplasias were also documented in several SIDS
cases. As such, maxillofacial deformities, which compromise the
upper airway, remain a hypothetical SIDS mechanism, as proposed
over three decades ago [55].
4.10. Carotid body abnormalities
In the postnatal period, the chemosensitivity of the carotid
body to hypoxemia gradually develops. Changes include proliferation of type I (chief) and II (sustentacular) cells, increased
numbers of dense core vesicles and K+ channels, modifications of
neurotransmitter/neuromodulator and receptor expression [56].
Thus, abnormalities of the carotid body structure and function
have been suggested to contribute to the pathogenesis of SIDS.
This was first reported in 1979 by Cole et al. who detected a
marked reduction or even the absence of the dense cytoplasmic
granules of the carotid chemoreceptor cells. He also noted a
reduction in cell number and size [57]. The authors postulated
that a defect in a respiratory control organ could block the
normal stimulation of respiration during the periods of hypoxia,
which occur during episodes of sleep apnea in infancy. These
results were not confirmed by subsequent research performing
light and electron microscopy of the carotid body from SIDS
victims compared to a control group of non-SIDS cases. In
addition, there were no differences among both groups in the
architecture, morphology and cellular mechanisms of neurotransmitter synthesis and storage [58]. However, the advent of
immunohistochemistry in recent years revealed a decrease in
type I cells and dense cytoplasmic granules and an increase in
progenitor cells in the carotid body of SIDS victims, which
suggest the immaturity of the carotid body [59]. Thus, through a
‘‘see-saw’’ process in the literature, the carotid body is once again
implicated to confer some underlying biological vulnerability in
some cases of SIDS.
Fig. 5. Posterior view of a volume rendered contrast-enhanced magnetic resonance
angiogram shows a left aortic arch with aberrant origin of the right subclavian
artery as last branch from the aortic arch (arteria lusoria) and common origin of the
common carotid arteries.
Courtesy: Dr. Christian J. Kellenberger, Diagnostic Imaging, University Children’s
Hospital, Zürich, Switzerland.
4.11. Aberrant cervical blood vessels
Rare congenital variations of the supra-aortic vessels were also
reported in SIDS victims. Arterial malformations include a common
carotid trunk, arteria lusoria (a rare abnormal variation of the right
subclavian artery which may cause a vascular ring around the
trachea and esophagus, Fig. 5) and an aberrant origin of the
vertebral arteries, from the common carotid artery on the right side
and from the aortic arch on the left. It may be that neck extension
and/or rotation causes vertebral artery compression and brain
stem ischemia in a few SIDS cases [60]. Rare venous malformations
in the neck, such as total anomalous pulmonary venous connection
(where the pulmonary venous circulation drains into the systemic
venous circulation rather than into the left atrium), were reported
to be occluded due to a fibrointimal hyperplasia in SIDS victims as
well [61].
5. Discussion
The first National Institute of Health definition of SIDS in 1969
required an autopsy of an infant who died in his sleep to rule out
other causes. This definition was debated over many years, and it
was revised by an expert panel of the National Institute of Child
Health and Human Development in 1991 [1]. The new definition
emphasized the necessity of autopsy, death scene investigation,
and review of the clinical history to provide accurate counseling to
parents. This change reflected one of the most significant lessons of
SIDS research: SIDS victims were not usually entirely normal
before death. Autopsies of SIDS victims enabled the documentation
of pathologies in crucial organs, such as ones in the head and neck
region. In 2004, the new definition of SIDS became ‘‘the sudden and
unexpected death of an infant under 1 year of age, with onset of the
lethal episode apparently occurring during sleep, that remains
unexplained after a thorough investigation, including performance
of a complete autopsy, and review of the circumstances of death
and the clinical history’’ [62].
Current data from the industrialized nations suggests that Japan
has the lowest reported SIDS rate (0.09 case per 1000 infants),
while New Zealand has the highest rate (0.80 per 1000), whereas
the US and the UK have an intermediate rate – 0.57 per 1000 in the
US [63], and 0.47 per 1000 for girls and 0.33 per 1000 live births in
boys in the UK [64]. Furthermore, there was a major decrease in
SIDS rates from 1990 to 2005 in 13 predominantly industrialized
countries. This decline may be attributable to diversity in how SIDS
is defined, as well as to trends in treatment options and increasing
awareness.
The head and neck region contains essential central organs,
which are involved in vital functions (Table 1). These organs can
play a relevant role in circumstances leading to a sudden death. It is
difficult to find consistent evidence to support the different
hypotheses in relation to the major risk factors for SIDS. SIDS is
almost certainly a multi-factorial and highly heterogeneous
disease and this is reflected by the multitude of hypotheses
concerning SIDS mechanisms and by numerous correlations that
have been reported between alterations in very diverse genes and
the occurrence of SIDS barring the emergence of a uniform image of
the disease. Autopsies are rare, and findings can be circumstantial.
SIDS hypotheses essentially revolve around defective respiratory and/or autonomical mechanisms. SIDS involves a convergence
of stressors that results in the asphyxia of a vulnerable infant who
has defective cardiorespiratory or arousal defense systems during
a critical developmental period when immature defense mechanisms are not fully integrated. Thus, our current understanding of
the pathogenesis of SIDS reflects the simultaneous convergence of
multiple factors that, when taken individually, are far less
detrimental than the result of their chance combination. SIDS
T. Marom et al. / International Journal of Pediatric Otorhinolaryngology 76 (2012) 311–318
Table 1
Head and neck pathologies reported in SIDS victims.
Pharynx
Elongated uvula
Lingual thyroglossal duct cyst
Hyper-secreting tonsils
Nasopharyngeal colonization with Strep pneumoniae
Larynx
Gastroesophageal reflux
Undescended larynx
Laryngotracheitis
Laryngeal airway obstruction
Ear
‘‘Silent’’ otitis media
Inner hair cells injury
Facial Skeleton
Back-set maxilla and mandible
Facial dysplasia
Neck
Carotid body abnormalities
Abberant right subclavian artery (arteria lusoria)
Aberrant vertebral artery
Pulmonary venous malformation
Brainstem
Hypoglossal nucleus abnormalities
remains a major problem that mandates continued interdisciplinary efforts for its ultimate resolution.
Clinical reports sum up in case reports or small series. Autopsy
guidelines differ worldwide and do not necessarily focus on head
and neck pathologies. Therefore, the level of evidence is low, since
there are no controlled, large-scale studies to support the findings.
The trends in the causes of SIDS have had a major impact on
surveillance and monitoring strategies. Since the apnea hypothesis
was common for many years, the use of different apnea monitors
has substantially increased, but yet it has not been shown to save
lives [65].
6. Conclusions
SIDS still remains an enigma in many aspects. In our view,
future SIDS research should focus on the autopsy evidences
collected so far, in order to establish a proactive risk-management
in high-risk infants. An emerging area of research which will likely
become the focus of future understanding is the hypoglossal
nucleus in the dorsal part of the medulla oblongata. It controls the
movement of the tongue, and in particularly the genioglossus
muscle, which is important in maintaining a patent airway,
especially during inspiration. There is higher incidence of
morphological pathological features of this nucleus in SIDS victims
when matched to control infants. In particular, the absence of gaminobutyric acid producing interneurons is noteworthy, which
interferes the sequential rhythmic activity of the motor neurons
and consequently the precise coordination of tongue movements.
This provides a potential anatomical substrate for respiratory and/
or swallowing failure and a neuroanatomical explanation for this
complex and fatal disorder [66].
Treatable disorders, such as extra-esophageal reflux, imminent
airway in recognized craniofacial and intrinsic congenital malformations which threaten it (by either primary excision or
tracheotomy) should be the addressed promptly, in addition to
other recommended cautions for this age group: sleep in the
supine position, sleep on a firm surface, keep soft objects and loose
bedding, avoid smoking, separate sleeping, consider offering a
pacifier and avoid overheating [67].
Financial disclosure
None.
317
Conflict of interests
None.
References
[1] M. Willinger, L.S. James, C. Catz, Defining the sudden infant death syndrome
(SIDS): deliberations of an expert panel convened by the National Institute of
Child Health and Human Development, Pediatr. Pathol. 11 (1991) 677–684.
[2] D.L. Hoyert, Mortality associated with birth defects: influence of successive
disease classification revisions, Birth Defects Res. A Clin. Mol. Teratol. 67
(2003) 651–655.
[3] H.C. Kinney, B.T. Thach, The sudden infant death syndrome, N. Engl. J. Med. 361
(2009) 795–805.
[4] B.M. Ostfeld, L. Esposito, H. Perl, et al., Concurrent risks in sudden infant death
syndrome, Pediatrics 125 (2010) 447–453.
[5] E.A. Mitchell, What is the mechanism of SIDS clues from epidemiology, Dev.
Psychobiol. 51 (2009) 215–222.
[6] D.C. Shannon, D.H. Kelly, SIDS and near-SIDS, N. Engl. J. Med. 306 (1982) 959–965,
1022–1028.
[7] J.J. Filiano, H.C. Kinney, A perspective on neuropathologic findings in victims of the
sudden infant death syndrome: the triple-risk model, Biol. Neonate 65 (1994)
194–197.
[8] M. Vennemann, T. Bajanowski, T. Butterfass-Bahloul, et al., Do risk factors differ
between explained sudden unexpected death in infancy and sudden infant death
syndrome? Arch. Dis. Child. 92 (2007) 133–136.
[9] J.P. Praud, P. Reix, Upper airways and neonatal respiration, Respir. Physiol.
Neurobiol. 149 (2005) 131–141.
[10] S.L. Tonkin, T.R. Gunn, L. Bennet, et al., A review of the anatomy of the upper
airway in early infancy and its possible relevance to SIDS, Early Hum. Dev. 66
(2002) 107–121.
[11] R.E. Stephens, A. Bancroft, A.G. Glaros, et al., Anatomic changes related to laryngeal descent from birth to 1 year of age: do they play a role in SIDS, Ear Nose
Throat J. 89 (2010) 313–317.
[12] S.E. Crelin, S.G. Scherz, Can the cause of SIDS be this simple, Patient Care 12 (1978)
234–241.
[13] P. Sidebotham, P. Fleming, Unexpected Death in Childhood: A Handbook for
Practitioners, John Wiley & Sons, England, West Sussex, 2007.
[14] L. Prtak, M. Al-Adnani, P. Fenton, et al., Contribution of bacteriology and virology
in sudden unexpected death in infancy, Arch. Dis. Child. 95 (2010) 371–376.
[15] J.R. Lucas, E.A. Haas, H. Masoumi, et al., Sudden death in a toddler with laryngotracheitis caused by human parainfluenza virus-1, Pediatr. Dev. Pathol. 12 (2009)
165–168.
[16] J.A. Morris, L.M. Harrison, S.M. Partridge, Practical theoretical aspects of postmortem bacteriology, Curr. Diagn. Pathol. 13 (2007) 65–74.
[17] M. Gleeson, R.L. Clancy, A.J. Cox, et al., Mucosal immune responses to infections in
infants with acute life threatening events classified as ‘near-miss’ sudden infant
death syndrome, FEMS Immunol. Med. Microbiol. 42 (2004) 105–118.
[18] A. Vege, T.O. Rognum, G. Anestad, IL-6 cerebrospinal fluid levels are related to
laryngeal IgA and epithelial HLA-DR response in sudden infant death syndrome,
Pediatr. Res. 45 (1999) 803–809.
[19] A.R. Highet, C.S. Gibson, P.N. Goldwater, Variant interleukin 1 receptor antagonist
gene alleles in sudden infant death syndrome, Arch. Dis. Child. 95 (2010)
1009–1012.
[20] J.P. Praud, Upper airway reflexes in response to gastric reflux, Paediatr. Respir.
Rev. 11 (2010) 208–212.
[21] Y. Yamamoto, Y. Atoji, Y. Suzuki, Innervation of taste buds in the canine larynx as
revealed by immunohistochemistry for the various neurochemical markers,
Tissue Cell 29 (1997) 339–346.
[22] J.T. Fisher, The TRPV1 ion channel: implications for respiratory sensation and
dyspnea, Respir. Physiol. Neurobiol. 167 (2009) 45–52.
[23] B.T. Thach, Reflux associated apnea in infants: evidence for a laryngeal chemoreflex, Am. J. Med. 103 (1997) 120S–124S.
[24] M. Al-Adnani, M.C. Cohen, I. Scheimberg, Gastroesophageal reflux disease and
sudden infant death: mechanisms behind an under-recognized association,
Pediatr. Dev. Pathol. 14 (2011) 53–56.
[25] R.E. Stark, S. Nathanson, Unusual features of cry in an infant dying suddenly and
unexpectedly, in: J. Bosma, J. Showacre (Eds.), Development of Upper Respiratory
Anatomy and Function: Implications for the Sudden Infant Death Syndrome., US
Printing Office, Washington, DC, 1975.
[26] R. Colton, A. Steinschneider, The cry characteristics of an infant who died of the
sudden infant death syndrome, J. Speech Hear Disord. 46 (1981) 359–363.
[27] M.P. Robb, D.H. Crowell, P. Dunn-Rankin, et al., Cry features in siblings of SIDS,
Acta Paediatr. 96 (2007) 1404–1408.
[28] R. Nachman, A. Krispin, M. Nnoli, et al., Infantile asphyxia due to aberrant uvula –
an anatomic misadventure, J. Forensic Leg. Med. 17 (2010) 401–403.
[29] Y. Kanawaku, M. Funayama, J. Sakai, et al., Sudden infant death: lingual
thyroglossal duct cyst versus environmental factors, Forensic Sci. Int. 156
(2006) 158–160.
[30] R.W. Byard, A.J. Bourne, M.M. Silver, The association of lingual thyroglossal duct
remnants with sudden death in infancy, Int. J. Pediatr. Otorhinolaryngol. 20
(1990) 107–112.
[31] P.S. Thrane, O. Rognumt, G. Brandtzaep, Sudden infant death syndrome (SIDS):
increased immune response in upper respiratory and digestive tract in SIDS,
Lancet 335 (1990) 229–230.
318
T. Marom et al. / International Journal of Pediatric Otorhinolaryngology 76 (2012) 311–318
[32] K.D. Forsyth, Immune and inflammatory responses in sudden infant death
syndrome, FEMS Immunol. Med. Microbiol. 25 (1999) 79–83.
[33] A.S. Hiller, A. Kracke, T. Tschernig, et al., Comparison of the immunohistology of
mucosa-associated lymphoid tissue in the larynx and lungs in cases of sudden
infant death and controls, Int. J. Legal Med. 110 (1997) 316–322.
[34] L. Stoltenberg, A. Vege, O.D. Saugstad, et al., Changes in the concentration and
distribution of immunoglobulin-producing cells in SIDS palatine tonsils, Pediatr.
Allergy Immunol. 6 (1995) 48–55.
[35] M. Gleeson, R.L. Clancy, A.J. Cox, et al., Mucosal immune responses to
infections in infants with acute life threatening events classified as ‘near-miss’
sudden infant death syndrome, FEMS Immunol. Med. Microbiol. 1 (42) (2004)
105–118.
[36] J. Blood-Siegfried, The role of infection and inflammation in sudden infant death
syndrome, Immunopharmacol. Immunotoxicol. 31 (2009) 516–523.
[37] S.J. Alter, N.K. Vidwan, P.O. Sobande, et al., Common childhood bacterial infections, Curr. Probl. Pediatr. Adolesc. Health Care 41 (2011) 256–283.
[38] M.M. Paparella, D. Shea, W.L. Meyerhoff, et al., Silent otitis media, Laryngoscope
90 (1980) 1089–1098.
[39] R.D. Eavey, Y.Z. Gao, H.F. Schuknecht, et al., Otologic features of bacterial meningitis of childhood, J. Pediatr. 106 (1985) 402–407.
[40] D.R. Djerić, P.A. Schachern, M.M. Paparella, et al., Otitis media (silent): a potential
cause of childhood meningitis, Laryngoscope 104 (1994) 1453–1460.
[41] Y. Hashimoto, J. Furumiya, Otitis media observed in unexpected natural death of
infants, Leg. Med. 11 (Suppl. 1) (2009) 121–123.
[42] J.B. Beckwith, Discussion of the terminology and definition of the sudden infant
death syndrome, in: A.B. Bergman, J.B. Beckwith, C.G. Ray (Eds.), Proceedings of
the Second International Conference on Causes of Sudden Death in Infants,
University of Washington Press, Seattle, (1970), pp. 14–22.
[43] J.F. Lubianca Neto, L. Hemb, D.B. Silva, Systematic literature review of modifiable
risk factors for recurrent acute otitis media in childhood, J. Pediatr. 82 (2006)
87–96.
[44] D.D. Rubens, B.R. Vohr, R. Tucker, et al., Newborn oto-acoustic emission hearing
screening tests: preliminary evidence for a marker of susceptibility to SIDS, Early
Hum. Dev. 84 (2008) 225–229.
[45] T. Allen, G. Juric-Sekhar, S. Campbell, et al., Inner ear insult suppresses the
respiratory response to carbon dioxide, Neuroscience 23 (175) (2011) 262–272.
[46] H.F. Krous, A.E. Chadwick, E. Haas, et al., Sudden infant death while awake,
Forensic Sci. Med. Pathol. 4 (2008) 40–46.
[47] A. Steinschneider, Prolonged apnea and the sudden infant death syndrome:
clinical and laboratory observations, Pediatrics 50 (1972) 646–654.
[48] S. Tonkin, Sudden infant death syndrome: hypothesis of causation, Pediatrics 55
(1975) 650–661.
[49] R.W. Byard, H.F. Krous (Eds.), Sudden infant death syndrome, Progress and
Possibilities, Arnold, Hodder Headline Group Problems, London, 2001.
[50] E.A. Mitchell, SIDS: past, present and future, Acta Paediatr. 98 (2009) 1712–1719.
[51] T. Dwyer, A.L. Ponsonby, Sudden infant death syndrome and prone sleeping
position, Ann. Epidemiol. 19 (2009) 245–249.
[52] A.L. Patel, D. Paluszynska, K.A. Harris, et al., Occurrence and mechanisms of
sudden oxygen desaturation in infants who sleep face down, Pediatrics 111
(2003) e328–e332.
[53] J.B. Beckwith, Observations on the pathological anatomy of the sudden infant
death syndrome, in: A.B. Bergman, J.B. Beckwith, C.G. Ray (Eds.), Proceedings of
the Second International Conference on Causes of Sudden Death in Infants,
University of Washington Press, Seattle, (1970), pp. 83–107.
[54] K. Rees, A. Wright, J.W. Keeling, et al., Facial structure in the sudden infant death
syndrome: case-control study, BMJ 317 (1998) 179–180.
[55] F. Cozzi, R. Albani, E. Cardi, A common pathophysiology for sudden cot death and
sleep apnoea. ‘‘The vacuum-glossoptosis syndrome’’, Med. Hypotheses 5 (1979)
329–338.
[56] E.B. Gauda, J.L. Carroll, D.F. Donnelly, Developmental maturation of chemosensitivity to hypoxia of peripheral arterial chemoreceptors – invited article, Adv. Exp.
Med. Biol. 648 (2009) 243–255.
[57] S. Cole, L.B. Lindenberg, F.M. Galioto Jr, et al., Ultrastructural abnormalities of the
carotid body in sudden infant death syndrome, Pediatrics 63 (1979) 13–17.
[58] D.G. Perrin, E. Cutz, L.E. Becker, et al., Ultrastructure of carotid bodies in sudden
infant death syndrome, Pediatrics 73 (1984) 646–651.
[59] A. Porzionato, V. Macchi, A. Parenti, et al., Peripheral chemoreceptors: postnatal
development and cytochemical findings in Sudden Infant Death Syndrome,
Histol. Histopathol. 23 (2008) 351–365.
[60] R. Pamphlett, J. Raisanen, S. Kum-Jew, Vertebral artery compression resulting
from head movement: a possible cause of the sudden infant death syndrome,
Pediatrics 103 (1999) 460–468.
[61] R.W. Byard, J.D. Gilbert, Total anomalous pulmonary venous connection: autopsy
considerations, Forensic Sci. Med. Pathol. V1-3 (2005) 215–220.
[62] H.F. Krous, J.B. Beckwith, R.W. Byard, et al., Sudden infant death syndrome and
unclassified sudden infant deaths: a definitional and diagnostic approach, Pediatrics 114 (2004) 234–238.
[63] R.Y. Moon, R.S. Horne, F.R. Hauck, Sudden infant death syndrome, Lancet 370
(2007) 1578–1587.
[64] National UK Statistics, Accessed 1 October 2011, http://www.statistics.gov.uk/
StatBase/Product.asp?vlnk=8660&More=Y.
[65] J.E. Hodgman, T. Hoppenbrouwers, Home monitoring for the sudden infant death
syndrome. The case against, Ann. N. Y. Acad. Sci. 533 (1988) 164–175.
[66] A.M. Lavezzi, M. Corna, R. Mingrone, et al., Study of the human hypoglossal
nucleus: normal development and morpho-functional alterations in sudden
unexplained late fetal and infant death, Brain Dev. 32 (2010) 275–284.
[67] American Academy of Pediatrics Task Force on Sudden Infant Death Syndrome,
The changing concept of sudden infant death syndrome: diagnostic coding shifts,
controversies regarding the sleeping environment, and new variables to consider
in reducing risk, Pediatrics 116 (2005) 1245–1255.