the solubility of compressed acetylene gas in tetrahydrofura
advertisement

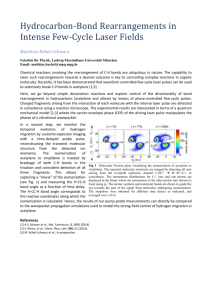
Title Author(s) Citation Issue Date URL The solubilities of compressed acetylene gas in liquids, IV : the solubility of compressed acetylene gas in tetrahydrofuran Kiyama, Ryo; Hiraoka, Hiroyuki The Review of Physical Chemistry of Japan (1956), 26(1): 1-8 1956-08-30 http://hdl.handle.net/2433/46734 Right Type Textversion Departmental Bulletin Paper publisher Kyoto University The Review of Physical Chemistry of Japan Vol. 26 No. 1 (1956) THE SOLUBILITIES OF COMPRESSEfl ACETYLENE GAS IN LIQUIDS, IV The Solubility of Compressed Acekylene Gas in Tetrahydrofuran* HY RYOKIYAMA ANDIlIROYUKI HIRAOKA Inkroduckion In the previous papers, the authors reported the solubilitiesof compressed acetylene gas in wa±er'>,methanol-' and benzene' and found that the solubility of acetylene in water at lugh pressures devia*.esnega±ively from Henry's law in the from N,=kP, where N: is the mole fraction of acetylene, P the partial pressure of acetylene and k a constant and the solubility is nearly proportional to the fugaci[y of acetylene, while the solubiHtiesin methanol and in benzene a± high pressures deviate positively from Henry's law and moreover the solution of benzene shows the positive deviation from Raoult's larv and this deviation cottld be well explained on the basis of the theory of regular solutions with the Flory-Huggins entropy correction arising from the difference in the nrolal volumes of the two substances. In the present paper, the solubility of acetylene in tetrahydrofuranhaving an electronegative oxygen atom in the molecule, a donor solvent for acetylene, in the range of temperature from 0 to 30°C and up to pressure 30 kg/cm', which has been never measured, is reported and rather qualitative considerations upon the characteristics of acetylene solutions are given. I Experimentals Tetrahydrofuran used as the solvent is fractionated by distillation, after refluxing with metallic sodium for several hours and a portion with boiling point 66.166.3°C (760mmHg) is taken, and its vapor pressure measured with an isoteniscope in the range of60 to 760mmHg and its density from 5 to 30°Care represented by Eqs. (1) and (2), respectively, dr=0.9079-9.93x10-"t-1.6x10-et (1) loge Pr=-1022x10-'+,5.635 .t+2~5 , (2) where P, is in mmHg and t ~indegrees centigrade. ` This incesiigation lrasbeendare by A. Itraoka, beingin fhe postgraduate course,underthe direcfiouof Prof.R. lcyarna. 1) H. Hiraoka,Thisjournal,24, 13 (1954) 2) R. Kiyamaand H. Hiraoka,ibid., 25, 16 (1955) 3) R. Kiyamaand H. H'vaoka,ibid., 25, 52 (1955) i The 2 Review of Physical Chemistry of Japan 1 (1956) Vol. 26 No. R. Kiyama and H. Hiraoka Acetylene used as the solute and the experimental measurements are all the same as those described procedures in the previous for the present reports. Resultr The vapor pressure and density of tetrahydrofuran the calculation of the solubility are represented of dilatation by the absorption the same valve the difference solvents of acetylene as in acetone'I among because the values are like one another,* at each temperature used in by Eqs. (1) and (2) and the coefficients in tetrahydrofuran the data in the other in tetrahydrofuran solvents and the correction are assumed are lacking and is comparatively of the solubility to have small if the due to the dilata+ion is small. Table 1 Solubility of acetylene in tetrahydrofuran at one atmosphere Temperature, °C Bunsen's coeH. 5 10 15 ?0 25 The solubility given in Table of acetylene 30.1 26.0 22.0 19.5 17.3 in tetrahydrofuran 1 and represented at one N_ denotes In Tables the mole fraction Table i of acetylene 2 and 3, the solubility ofcompressed is given in the mole fraction 2 pressure is by Eq. (3), log,° N_=8.85~x 10'_4.155 where atmospheric of acetylene and T the absolute acetylene and the numbers Solubility of zcetylene (3) temperature. gas in tetrahydrofuran of cc of acetylene (S. T. P.) in tetrahydrofuran (mole fraction of acetylene, N_) Temp. Press., °C 0 10 20 30 5 U.341 0.237 0.231 0.192 10 0.519 0.439 0.369 0.323 0.491 0.433 0.595 0.532 0.704 0.613 kglcmp 15 I ?0 S s acetylene 4) 0.691 This will be confirmed in [he following paper in which tbe partial in various solvents will be reported. J. Horiuti, Sri. Posers Inst. Phys. Citem, Resemch, Tokyo, ]7. 125 (1931) molal volumes of The The Solubilities Table 3 oI Compressed Solubility Temp., Review of Physical Chemistry Acetylene Gas in Liquids, of acetylene of Japan Vol. 26 No. IV 3 in tetrahydtofuran °C a ~.a 1~ 112 296 217 Press., kg/cm= 5 10 ~.~ 15 617 20 25 o.~ 64~ ~~ 129 264 207 402 307 649 929 25kB'°" i.e zOk to C os 15k8/~^' i ~~ L6 10 1A ykflj 'C TN n O.a O C 20'C 2` s oa i2 C 0g ~ 1.0 ~a~` 0.7 ~.8 0 5 10 15 2(1 Partial p¢ssurc of acetykoa kg/cm' 33 Fig. L Solubility of acetylene in tetrahydrofuran as function of partial pressure of acetylene contained fraction that in solubility N.,=kP logarithm of temperature gation. has of of acetylene the form 1 cc been made is plotted at high and Fig. the mole at The tetrahydrofuran, each of of Dalton's gaseous phase calcular_ed is described later- the linear and according the the to the method 1, from in which fraction partial of developed of the mole it is observed Henry's law exists between reciprocal range to the mole Fig. relationship the temperature pressure and 3.6 of acetylene, negatively acetylene total law, In a nearly within 35 I /T ~10' Logarithm of mole fraction of acetylene ploited against reciprocal of absolute temperature thepartialpressure deviates that pressure conversion using against fraction Fig. 2 respectively. pressures 2 shows 3A of the the present pressure tetrahydrofuran by Robin in the the absolute investi- of acetylene in the of nl., which 1 (1956) V The 4 Review of Physical Chemistry 1 (1956) of Japan Vol. 26 No. in water, methanol, R. Ki}ama and H. Hiraolsa Considerations The benzene authors have measured and tetrahydrofuran view of the effect of the the and these partial the solubility at high pressures the case in whichthe in benzene latter, belong as is seen against solubility in water and solubility the solubilities pressure deviates nonpolar to the former positively Fig. 3 in which the pressure of .acetylene. is exceptionally donor solvents mole fraction or in water that belongs the mole fraction highest when the in and the is plotted that the of acetylene those in these in water of acetylene and to the it is notable to the fugacity among other in methanol of acetylene case. of acetylene solubility to the former to the latter, law and in tetrahydrofuran In the latter is the into two cases One is the case in which The solubilities low and proportional in tetrahydrofuran in general classified from Henry's and those in water It may be considered solvents aze of acetylene. used and fifty times as high as the mole fraction 20 kg/cm=. of acetylene solubility :deviates negatively. from the. partial solubilities solvents at 20°C and in alcohol or in and the solubility of acetylene solubility is represented in by the of acetylene. 0-~ hfethanol os TecrahYdMfuran 0.5 Water ti 2 OS ~_os y Bemene Y bT Q Q Pamrene ` o =' 0.4 ` c .~03 <` Sfethanol Tetrahvdrofuran 0.2 Wafer os io t; zo zs an partialDressure of acetylene: kgrem' Fig. 3 Solubility of acetylene as function of partial pressure of acetylene at ~°C Now, pure chosen as the standard acetylene ratio liquid acetylene of the fugacity The futLacity of pure L state in the liquid solution in equilibrium of acetylene in equi]ibrium of acetylene acetylene in the is already o oz o.a ob oa h7olefranionof acetykne..h4 Fig. 4. Activity of acetylene plotted against mole fraction of acetylene at 20°C with its vapor at each temperature in the liquid solution, then the activity with the gaseous soluton reported is of phase is equal to the [o that in the pure liquid state. and the concentration of the J The Review of Physical Chemistry of Japan The SolubIli[iesof Compressed AcetyleneGas in Liquids, IV Vol. 26 No. 1 (1956) 5 liquid solvent in the gaseous phase necessary to the calculation of the fugacity of acetylene in solutions is assumed to be approximately computed by Eq. (4) according to Robin et al.~, log~n ~-(A+131T)P ° (4) where m is the mass of the liquid solvent per 1 cc of the gaseous phase of density p in Amagat unit and ara° the mass of the liquid solvent contained in 1 cc of the gaseous phase in the absence of the compressed gas. The constants in Ey. (4) are shown in the table. System acetylene-water acetylene-methanol acetylene-benzene acetylene-tetrahydrofuran A -0.00187 -0.00234 -0.00544 0.00160 A 2.45 2.01 5.75 I ~ In [he last system, acetylene-tetrahydrofuran. the first term A on the right hand side of Eq. (4) contains only the contribution by the Poynting effect, and the second term is taken into no account because the appropriate physical constants of Letrahydrofuran necessary to the calculation are unavailable. Then the fugacity of acetylene in solutions in equilibrium with the gaseous phase is calculated as an approximation using Lewis-Randall's law, f =f_°N_, where. f., is the fugacity of acetylene in the solution and f_° the fugacity in the pure state. 1'he difference between the value of the fugacity thus obtained and the value under the assumption that the gaseous phase is pure acetylene is considerable at high temperatures and low pressures, but the difference is only slight at low temperatures and high pressures. Fig. 4, in which the activity of acetylene in each solution at 20°(; is plotted against the mole fraction of acetylene, shows [hat the positive deviations from Raoult's law are found in the soiution of acetylene in water, methanol and benzene, whereas the negative deviation in the solution of acetylene in tetrahydrofuran, In the discussion of the solutions of compressed gases, the change of free energy or activity with pressure mus*_be taken into account and this change is related by the well known thermodynamic equa~Son to the partial molal volume of the solute in solutions. In the following paper the authors will report on the measurements of the partial molal volume of acetylene in the wide range of concentration in the various solvents. So, in the present paper qualitative considerations about the characteristics of acetylene solutions may be rather given in the following. Acetylene-toater system As mentioned previously, the solutions of acetylene in wa*.er, alcohol and nonpolar solvents, such as benzene, deviate positively from Raoult's law. Among t4zm, aqueous 5) S. Robin et B. Vodar, J. Fhys. e! Fad.,.13, 264 (1952) i i The 6 Review of Physical Chemistry of Japan Vol. 26 No. R. Kiyama and H. Hiraoka solution shows the . activity with each the lazgest against other positive the mole but the deviation fraction activity and Fig. 5 indicates at 20°C and 30°C are at a low temperature that Imear gradually the isotherms and nearly decreases of coincide from the 37 K 3UC O.fi v temperature _T 1°C 10°C o Q4 T 20°C 0 30°C G i .~ os 1'C ~a~i 0 OAW OA10 0.015 0~2D ttolo fraztionof acetylene,,~, Fig. 5 Activity of acetylene in water plotted against mole fraction of acetylene values at 20°C and 30°C, water is negative, These indicate that the heat of sohttion of liquid acetylene that is, exothermic has a lazge negative value. and the excess partial molal entropy The assumption°I• ~1 that the rare gases in of solution and hydrocarbon gases form "iceberg" when they dissolve in cold water and that those molecules in solution may be surrounded by oriented cages of water molecules, gives a fine interpretation for the characteristics of aqueous solution of acetylene and that the isotherms at temperatures higher than the critical temperature of acetylene, which is obtained and that of the gas hydrate the isotherms supposed partial to be large Acetylene-metlrauol and is equal to 16°C°~, would molal are drawn heat capacity of acetylene neazly coincide, lower with decreasing of acetylene whereas temperatures in aqueous solution is at lower temperatures. system Acetylene•methanol result of various pressures difference from the cross point of the vapor pressure at lower temperatures and from this the it is ohserved of the hydrate factor, system shows a positive deviation the association of methanol, the from Raoult's law, as a net difference in the internal of the two substances, the interaction of methanol and acetylene, the in the molal volumes of the two substances and so on. The temperature 6) H. S. Frank and M. N. Evans, J. Chem.Phys., 13, 507 (19x5) 7) W. F. Clauesen and M. F. Polglase, J. Am. Chem. Soc., 74, 9817 (1952) 8) M. V. Stackelberg and H. R. Muller, Zeit. Efectrachemie, 58, 25 (1954) 1 (1956) a The The Solubilities coefficient so that of the the activity Mcintosh's and alcohol benzene at lots is, endothermic of solution condensation is positive, in methanol of Japan Vol. 26 No. 1V as from the heat gaseous of to other the from of the temperature of solution gas. It solutions in may Raoult's shown in Fig. 6, in accordance compound of acetylene be of this deviation and with of molal the acetylene of regular benzene solution solutions in the liquid theory of acetylene the law regular coefficient of tvi*1t the acetylene that differences is of considered activity of in benzene course could of the be two acetylene is positive, although exothermic this in Flory•Huggins volumes solutions, that in nonpolar the acetylene the owing is that heat to the interpretation may be solvents. %' 0 P/ os so ~~ .•~/ as as ~r: ~/ ad 0 OA OA s ~.; .'T 0 :'~ 0'C 02 Acetone 02 Mok fraMionof aoetykoe. N 0 Fig. 6 02 Activity against OA of acetylene mole fraction Ob in methanol solutim at 3C Mole (racoon of acetylene, N. OS 0 plotted Fig. 7 of acety]ene 02 OA 0.6 • x 0°C 10°C • x temperature 0°C ]0°C p O ZO°C 30°C ~ O 20°C 30°C D. Mcintosh, j. Phys. Chem., 11, 306 (1907) OB Activity of acetylene in tetrahydrofuran plotted against mole fraction of acetylene temperature 9) 1 (1956) 7 is negative of a crystalline reported theory in accordance of applicable the arising the authors deviation of Moreover so that the positive basis corrections negative, acetylene of the formation paper, a on the substances. Gas in Liquids, in methanol of liquid Chemistry sysfe»z shows entropy Acetylene of Physical temperatures. previous explained acetylene observation's Acetylene-benzene In the of hear_ of soluraon with of Compressed Review i I The S Review of Physical Chemistry of Japan Vol. 26 No. R. Kiyama and H. Hiraoka Acetylene•tetrahydrafiaran Tetrahydrofuran molecule sys[en: has an electronegazive which has electrons the proton of acetylene. having an active center that are Studies oxygen available atom in the carbon for hydrogen on the solubilityof acetylene have been made extensively bond chain of the formation with in such a donor solvent from the technical point of view, but those under high pressures are comparatively few. 1'he solubility of acetylene in acetone measm~ed by Holemann ei a1,.10>changes in the same manner as in tetrahydrofuran with pressure. Fig. 7, in which the activity of acetylene acetylene, acetylene obtained activity shows that in tetrahydrofttran from the data of acetylene tion between by Holemann of hydrogen The authors for the Fundamental against Raoult's the than that in the indicating that is, exothermic solvent molecule bond formation mole fraction solution of acetone coefficients and the strong specific interacmay be expected between in accordance the electronegative atom of acetylene. has been done by Mr. N. Yoshida. to the Department Scientific Individual Research of Education for the Grant (1'he Yhysico-Chemical The Laboratory of Physical Kyoto University and R. Ilasselmann. of the that the heats of solution Clrem. ing. Teclmik, 25, 4ti6 (1953) in Aid Researches on Acetylene). 1D) P. Holemann of law in the solution of e! al. and the temperature measurements are indebted smaller are positive, in the donor solvent and the proton Part of the present I is slightly and donor is plotted de~~ation from are negative, acetylene with the assumption negative in both solvents in such donor solvents i the Chemistry, 1 (1956)