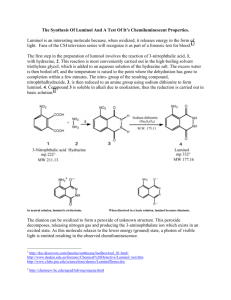
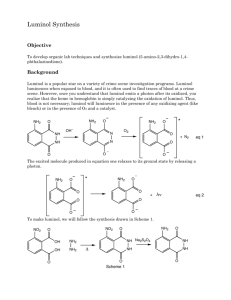
Luminol Synthesis and Chemiluminescence Experimental
advertisement

Luminol Synthesis and Chemiluminescence Background Information. See Wikipedia: http://en. wikipedia .org!wiki/Luminol Experimental Procedure Safety Notes! Use protective gloves. Conduct all operations using the following chemicals in a fume hood. Hydrazine is toxic and a suspected human carcinogen. Potassium hexacyanoferrate (III) is light-sensitive and dangerous when heated as a solid and formation of lethal HCN is possible if a solution comes into contact with ammonia, chromic acid, sodium nitrite, or strong acids. Sodium hydrosulfite (sodium dithionite, Na2S2O4) is flammable and moisture sensitive. NaOH is corrosive. In case of contact with skin, flush with copious amounts of water. Luminol and 1,2-propanediol are irritants. Avoid breathing vapors and contact with the skin. Use in a well-ventilated area only. In case of accidental contact, flood the affected area with copious amounts of water. All materials must be discarded in the containers provided. Initial Preparation 1) Place sand -a depth of at least 3 inches -in a heating well, and insert a thermometer, held by a clamp, into the sand. Bring the temperature of the sand to 200 °C. 2) Using a hot plate, heat 25 mL of distilled water in a 100 mL Erlenmeyer flask to near boiling. 3) Fill a 250 mL beaker with an ice-water mixture. Cool 5 -10 mL of distilled water in a test tube using this ice water bath. Synthesis of Luminol 4) Put 0.5 g of 3-nitrophthalic acid in a large test tube, then add 2.0 mL of propylene glycol and then add 0.5 mL of 20% aqueous hydrazine solution. 5) Add a boiling chip and immediately immerse the test tube in the 200°C sand bath to a depth that heats about half of the test tube. Hold the test tube in the sand bath with a clamp, and insert a thermometer into the reaction mixture to permit you to directly monitor its temperature. 6) When the reaction mixture begins to boil, note its temperature in your lab notebook, and continue heating until the reaction mixture reaches 150 °C. Continue heating to maintain the reaction temperature at 150 °C for another 15 min. 7) Remove the reaction mixture from the sand bath, allow the mixture to cool to 100°C, and then add 3 mL of the hot distilled water. Written by John McCormick at the University of Missouri-Columbia 8) Cool the diluted reaction mixture in an ice-water bath for 5 -10 min, scratching with a glass rod to induce crystallization if necessary. 9) Collect the crystals by vacuum filtration using a Hirsch funnel. Break the vacuum, add 2 -3 mL of icecold distilled water to rinse the crystals, and then remove the wash water using a vacuum. Note the color of the crystals in your lab notebook. 10) Using a spatula, collect the crystals and place them in the test tube you used for their synthesis. Add 3.0 mL of 5% NaOH solution. Note the color of the solution in your lab notebook. 11) Then add 1.0 g of sodium hydrosulfite (sodium dithionite, Na2S2O4) and heat the reaction mixture in the sand bath for 3 minutes. Note the color of the solution in your lab notebook. 12) After the 3 min heating period, remove the test tube from the sand bath and add 1.0 mL of glacial acetic acid. Use a stirring rod to mix the reaction mixture well. 13) Cool the test tube in the ice-water bath for 5 -10 min, and then collect the luminol crystals by vacuum filtration using a clean Hirsch funnel. Break the vacuum, rinse the crystals with 2 -3 mL of chilled distilled water, and remove the rinse water using a vacuum. 14) Pull air through the crystals briefly, and then weigh the moist luminol to obtain a crude yield weight. Note the color of the crystals in your lab notebook. Chemiluminesence 15) Set the Hirsch funnel on top of a 250-mL Erlenmeyer flask and put your luminol crystals in the Hirsch funnel. Dissolve the crystals by pouring 15 mL of 5% NaOH solution through the funnel, collecting it in the Erlenmeyer flask. Finish by rinsing the funnel with 5 mL of distilled water, which also should be collected in the Erlenmeyer flask. Then dilute the solution by addition of 100 mL of distilled water. This is your luminol solution for the chemiluminesence reaction. 16) To carry out the chemiluminesence reaction, place 25 mL of luminol solution in one Erlenmeyer flask, and 25 mL of oxidizing solution in a second Erlenmeyer flask. Place a standard funnel on top of a 125-mL Erlenmeyer flask. In a darkened area, simultaneously pour the two solutions into the funnel, noting the exact time. Gently swirl the flask. In your notebook, record the color and the duration of the chemiluminesence. You may repeat the chemiluminesence reaction -using varying (equal) amounts of the two solutions as you wish -until the luminol solution is exhausted. Clean up Discard all wastes in the appropriately labeled containers. No organic materials from this lab go down the drain in the sink! After you have poured all you can into the waste containers, wash glassware containing only residual amounts (clinging to the sides of the glassware) in the sink. Written by John McCormick at the University of Missouri-Columbia