Unit 6
Energy Transfer in Earth
Systems
• Chapter 11 ~ Energy in Earth’s Atmosphere
o Section 1 ~ The Air Around You
o Section 2 ~ Behavior of Gases
o Section 3 ~ The Layers of The Atmosphere
• Chapter 12 ~ Weather & Climate Factors
o
o
o
o
o
Section 1 ~ Energy in the Atmosphere
Section 2 ~ Winds
Section 3 ~ Water in the Atmosphere
Section 4 ~ Air Masses and Fronts
Section 5 ~ Climate
• Chapter 13 ~ Forces in Nature
o Section 1 ~ Weathering, Erosion, and Deposition
o Section 2 ~ Mapping the Surface
Unit 6 covers the following framework standards: ES 3, 4, 6 and PS 16. Content was adapted the following:
Prentice Hall (Ed.). (2009). The Science Explorer: Weather and Climate. Boston,
MA: Pearson.
Prentice Hall (Ed.). (2009). The Science Explorer: Earth’s Changing Surface. Boston,
MA: Pearson.
McLaughlin, C. W., & Thompson, M. (1999). Physical science. Columbus, Ohio/USA:
Glencoe/McGraw-Hill.
94 Chapter 11 The Energy in Earth’s Atmosphere
Section 11.1
The Air Around You
Terms:
• Weather
• Atmosphere
• Climate
• Ozone
• Water Vapor
Weather is the condition of
Earth’s atmosphere at any
given
moment.
For
instance,
during
the
summer in Massachusetts the weather may be dry and warm in the morning and
then become wet and cooler after a thunderstorm! The atmosphere is the
envelope of gases that surrounds the planet. To understand the relative size of
the atmosphere, imagine that Earth is the size of an apple. If you breathe on the
apple, a thin film of water droplets will form on its surface. Earth’s atmosphere is
like that water on the apple—a thin layer of gases on Earth’s surface.
The weather in an area changes every day. Climate, on the other hand, refers to
the average, year-after-year conditions of the temperature, precipitation, winds,
and clouds in an area. For example, California’s Mojave Desert, has a hot, dry
climate. Scientists use two main factors—precipitation and temperature—to
describe the climate of a region. A climate region is a large area that has similar
climate conditions throughout. As this chapter focuses on the energy transfer
within Earth’ systems, it will at times relate to weather patterns and causes of
climate.
Composition of the Atmosphere The atmosphere is made up of a mixture of
atoms and molecules of different kinds. An atom is the smallest unit of a
chemical element that can exit by itself. Molecules are made up of two or more
atoms. Earth’s atmosphere is made up of nitrogen, oxygen, carbon dioxide,
water vapor, and many other gases, as well as particles of liquids and
solids. The air around you contains particles of dust, smoke, salt, and other
chemicals, but most of them are too small to see.
95 Nitrogen is the most abundant gas in the
atmosphere, making up a little more than three
fourths of the air we breathe. Each nitrogen
molecule contains two nitrogen atoms. Oxygen
is the second most abundant gas in the
atmosphere, making up about 21% of the
volume. Plants and animals take oxygen
directly from the air and use it to realize energy
from their food. Oxygen is also involved in
many other processes like fuel used to light a
fire. Oxygen also reacts with steel in cars and
other objects to form iron oxide, or rust.
Most oxygen molecules have two oxygen
atoms. Ozone is a form of oxygen that has
three oxygen atoms in each molecule instead
of the usual two. Have you ever noticed a
pungent smell in the air after a thunderstorm?
This is the odor of ozone, which forms when
lightning interacts with oxygen in the air.
Carbon dioxide and argon make up less than one percent of the gases in the
atmosphere. Each molecule of carbon dioxide has one atom of carbon and two
atoms of oxygen. Carbon dioxide is essential to life. Plants must have carbon
dioxide to produces food. When the cells of plants and animals break down food
to produce energy, they give off carbon dioxide as a waste product. When fuels
such as coal and gasoline are burned, they release carbon dioxide. Burning
theses fuels increases the amount of carbon dioxide in the atmosphere.
Water Vapor
So far, we have discussed the composition of dry
air. In reality air is not dry because it contains water vapor. Water vapor is water
in the form of a gas. Water vapor is invisible. It is not the same thing as steam,
which is made up of tiny droplets of liquid water. Each water molecule contains
two atoms of hydrogen and one atom of oxygen. The amount of water vapor in
the air varies greatly from place to place and from time to time.
Water vapor plays an important role in Earth’s weather. Clouds form when water
vapor condenses out of the air to form tiny droplets of liquid water or crystals of
ice. If these droplets or crystals become heavy enough, they can fall as rain or
snow.
Importance of the Atmosphere Earth’s atmosphere makes conditions on Earth
suitable for living things by contains oxygen and other gases that living things
96 need to survive. In turn, living things affect the atmosphere. The atmosphere is
constantly changing, with gases moving in and out of living things, the land, and
the water. Also, living things need warmth, and liquid water. By trapping energy
from the sun, the atmosphere keeps most of Earth’s surface warm enough for
water to exist as a liquid. In addition, Earth’s atmosphere protects living things
from dangerous radiation from the sun. The atmosphere also prevents Earth’s
surface from being hit by most meteoroids, or rocks from outer space.
Summary:
• Earth’s atmosphere is the covering of gases that surrounds Earth.
Compared to the size of the Earth, the atmosphere is a very thin
covering. The atmosphere is made up of about three-fourths nitrogen,
one-fourth oxygen, and a small amount of other gases, as well as
particles of liquids and solids.
• The atmosphere is commonly called the air.
• Most oxygen molecules in the atmosphere have two oxygen atoms, but
another form, called ozone, has three oxygen atoms.
• The amount of water vapor in the atmosphere varies. Water vapor is the
gas form of water.
• Weather is the condition of the atmosphere at a particular time and place.
For example, the weather you have right now is the condition of the
atmosphere at your particular place.
97 Section 11.2
Behavior of Gases
Terms:
• Pressure
• Pascal
Pressure
Every time you feel the wind of your face, you
observe the behavior of a gas—rather, the mixture of gases that is Earth’s air, or
atmosphere. Even when the wind is calm, the air exerts a force called pressure.
What causes the pressure of a gas? Particles of matter are small—many billions
of particles of air fill an inflated toy balloon. When riding on a bike, you’re riding
on pockets of colliding air particles inside your tires. You have learned that the
particles of air, like those in all gases, are constantly moving. They’re free to fly
about and collide with anything in the way. The collisions with the inside walls
keep the moon or tires inflated and cause the force that you feel when you
squeeze them. The total amount of force exerted by a gas depends on the size of
its container. Pressure is the amount of force exerted per unit of area.
Measuring Pressure
The pascal (Pa) is the SI unit of pressure. One
pascal of pressure is a force of one newton per square meter. This is a small
pressure unit, so most pressures are given in kilopascals (kPa). Earth’s
atmosphere exerts pressure on everything within it. At sea level, atmospheric
pressure is 101.3 kPa. This means that at Earth’s surface, the atmosphere exerts
a force of about 100,000 N on every square meter. This amount of force is equal
to a eight of 100,000 N—about the weight of a large truck.
Our Atmosphere
You can see right through it, and most of
the time you can’t even feel it. But the air we breathe and live in contains many
gas particles that cause air pressure. These particles move so fast, approaching
1610 km/hr, that one particle may collide with another, or you, as often as every
billionth of a second. Within the troposphere, millions of fast-moving colliding
particles create air pressure in every square meter.
Higher Altitudes Mean Less Pressure
Above the troposphere, there are
fewer gas particles and fewer collisions, which means less pressure. The
stratosphere continues to about 50 km above Earth’s surface. At higher
distances, the mesosphere and thermosphere continue. If the air pressure is
measured at 5km above Earth, the pressure will have dropped from 101 kPa at
Earth’s surface to approximately 54 kPa. At 50 km, the pressure may be only
0.15 kPa. An example of the effect of altitude on pressure is shown by the water
bottle in the figure.
98 Boyle’s Law
Suppose you have some gas in a sealed flexible container, such as a balloon.
You can squeeze or stretch the container without changing the amount of gas
trapped inside. The pressure of a gas depends on how often its particles strike
the walls of the container. If you squeeze some gas into a smaller space, its
particles will strike the walls more often, giving an increased pressure. This
behavior explains why when you squeeze a balloon into a smaller space, it
causes the balloon to push back with more forces. The reverse happens, too. If
you give the gas particles more space, they will hi the walls left often and the gas
pressure will be reduced. Robert Boyle (1627-1691), a British scientist, described
this property of gases. According to Boyle’s law, if you decrease the volume of a
container of gas, the pressure of the gas will increase, provided the temperature
does not change. Increasing the volume causes pressure to drop. As you’ll see, it
is important that the temperature remains constant.
Charles Law
IF you’ve seen a hot-air balloon being inflated, you know gases expand when
heated. Jacques Charles (1742-1823) was a French scientist who studied gases.
According to Charles’s Law, the volume of a gas increases with increasing
temperature, provided the pressure does not change. As with Boyle’s law, the
reverse is true, also. The volume of a gas shrinks with decreasing temperature.
Using his law, Charles was able to calculate the temperature at which a gas
would have a volume of zero. The kinetic theory would say that this sit eh
temperature at which all particle motion of matter should stop. Charles found this
temperature to be -273 C, or 0 K, also called absolute zero. In realty, gases
cannot be cooled to zero volume. Instead, they condense to liquids when cooled
below their boiling points.
You can explain Charles’s law by using the kinetic theory of matter. As a gas is
heated, its particles move faster and faster and its temperature increases.
Because the gas particles move faster, they begin to strike the walls of their
container more often and with more force. If the walls are free to move, the gas
pushes the walls out and expands.
Summary
• Main Idea: There are more than 100 elements that combine in a multitude
of ways to produce compounds that make up all of the living and
nonliving things that we encounter.
• Gas pressure is caused by moving particles colliding with the inside walls of its
container.
99 • Boyle’s law states that the volume of a gas decreases when the pressure
increases, at constant temperature.
• Charles’s law sates that the volume of a gas increases when the temperature
increases, at constant pressure.
100 Section 11.3
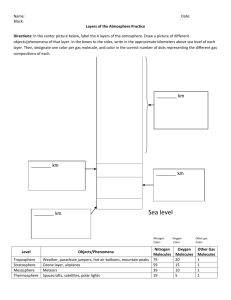
Layers of the Atmosphere
Terms:
• Troposphere
• Mesosphere
• Stratosphere
• Thermosphere
• Ionosphere
• Exosphere
Imagine you are taking a trip upward into the atmosphere in a hot-air balloon.
You begin on a warm beach near the ocean, at an altitude of 0 kilometers above
sea level. As the balloon rises to an altitude of 3 kilometers, you realize that the
air is getting colder. As you continue to rise, the air gets colder still. At 6
kilometers you begin to have trouble breathing because the air is becoming less
dense, so you start to go back down.
If you continued to ride up through the atmosphere, the air pressure and
temperature would change dramatically. Scientists divide the Earth’s atmosphere
into four main layers classified according to changes in temperature. These
layers are called the troposphere, the stratosphere, the mesosphere, the
thermosphere. The thermosphere is also divided into two layers: the ionosphere,
and exosphere. Refer to the diagram as you read about each layer.
The Troposphere
You live in the inner, or lowest, layer of the
Earth’s atmosphere, the troposphere. Tropo- means, “changing.” Conditions in
the troposphere are changing because this is the layer of that atmosphere in
which Earth’s weather occurs. The depth of the troposphere varies from 16
kilometers above the equator to less than 9 kilometers above the North and
South poles. Although it is the shallowest layer, the troposphere contains almost
all of the mass of the atmosphere! As altitude increases in the troposphere, the
temperature decreases. On average, for every 1-kilometers increase in altitude,
the air gets about 6.5 Celsius degrees cooler. At the top of the troposphere, the
temperature stops decreasing and stays at about -60° C. Water here forms thin,
feather clouds of ice.
The Stratosphere
The stratosphere extends from the top of
the troposphere to about 50 kilometers above Earth’s surface. Strato- means,
“spread out.” The stratosphere is the second layer of that atmosphere and
contains the ozone layer. The lower stratosphere is cold, about -60° C.
Surprisingly, the upper stratosphere is warmer than the lower stratosphere. Why
101 is this? The middle portion of the stratosphere contains a layer of air where there
is much more ozone than in the rest of the atmosphere. (Recall that ozone is the
three-atom form of oxygen.) When the ozone absorbs energy from the sun, the
energy is converted into heat, warming the air. The ozone layer is also important
because it protects Earth’s living things from dangerous ultraviolet radiation from
the sun.
The Mesosphere
Above the stratosphere, a drop in temperature
marks the beginning of the third layer, the mesosphere. Meso- means “middle,”
so the mesosphere is the middle layer of the atmosphere. The mesosphere
begins 50 kilometers above Earth’s surface and ends at an altitude of 80
kilometers. In the outer mesosphere, temperatures approach -90°C. The
mesosphere is the layer of the atmosphere that protects Earth’s surface from
being hit by most meteoroids. Recall, meteoroids are chunks of rock from space.
When you see a shooting star, you are seeing the glowing gases that a meteor
leaves behind in the mesosphere!
The Thermosphere
Near the top of the atmosphere, the air is very
thin. At 80 kilometers above Earth’s surface, the air is only about 0.001 percent
as dense as the air at sea level. It’s as though you took a cubic meter or air at
sea level and expanded it into 100,000 cubic meters at the top of the
mesosphere. The outermost layer of Earth’s atmosphere is the thermosphere.
The thermosphere extends from 80 kilometers above Earth’s surface outward
into space. Thermo- means “heat.” Even though the air in the thermosphere is
thin, it is very hot, up to 1,800° C. This is because sunlight strikes the
thermosphere first. Nitrogen and oxygen molecules convert this energy into heat.
Despite the high temperature, you would not feel warm in the thermosphere. An
ordinary thermometer would show a temperature well below 0°C. Why is that?
Temperature is the average amount of kinetic energy, or motion of each
molecule of a substance. The gas molecules in the thermosphere move very
rapidly, so the temperature is very high. However, the molecules are spaced far
apart in the thin air. There are not enough of them to collide with a thermometer
and warm it very much.
The thermosphere is divided into two layers. The lower layer, called the
ionosphere, begins about 80 kilometers above the surface and extends to about
400 kilometers. Energy from the sun causes gas molecules in the ionosphere to
become electrically charged particles called ions. Radio waves bounce off ions in
the ionosphere back to Earth’s surface. Brilliant light displays, called the Northern
Light, or the aurora borealis. Auroras are cased by particles from the sun that
enter the ion sphere near the poles. These particles strike atoms in the
ionosphere, causing them to glow.
102 Exo- means “outer,” so the exosphere is the outer most portion of the
thermosphere. The exosphere extends from about 400 kilometers outward for
thousands of kilometers.
103 Summary:
• The atmosphere is divided into layers based off of temperature changes.
They layers include the troposphere, stratosphere, mesosphere, and
thermosphere. The thermosphere contains two layers: the
ionosphere and the exosphere.
• We live in the troposphere. The troposphere is the layer of the
atmosphere in which Earth’s weather occurs. It also contains almost
all of the mass of the atmosphere. As altitude increases, the
temperature decreases. Meaning, the higher you go up, the colder it
gets.
• The stratosphere is the second layer of the atmosphere and contains the
ozone. Temperatures increase with altitude in the stratosphere
because the ozone absorbs radiation from the sun.
• The mesosphere is the layer of the atmosphere that protects Earth’s
surface from being hit by most meteoroids. The temperatures
decrease with altitude. Meteoroids that enter this layer burn up and
we see the left over glowing gases as a “shooting star”.
• The thermosphere is the outermost layer of the Earth’s atmosphere and
contains two layers: the lower layer called the ionosphere where
colorful displays called the Northern Lights take place, and the outer
layer called the exosphere. The exosphere extends far out into outer
space. Temperatures increase with altitude in the thermosphere
because the sun’s energy hits this layer first. However, the molecules
are so spread out that it feels cold in the thermosphere.
104