A1 Bonding WS1 Ans
advertisement
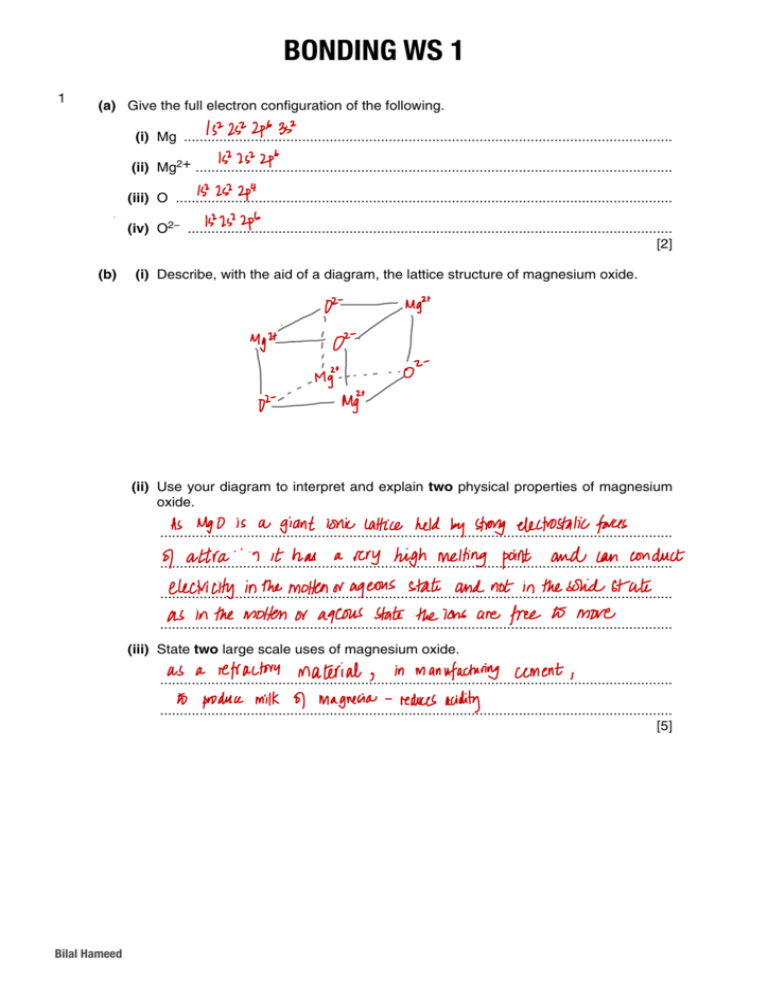
BONDING WSWS1 3 BONDING 2 11 1 For Examiner’s Use (a) Give the full electron configuration of the following. (i) Mg ............................................................................................................................ (ii) Mg2+ ......................................................................................................................... (iii) O .............................................................................................................................. (iv) O2– ........................................................................................................................... [2] (b) (i) Describe, with the aid of a diagram, the lattice structure of magnesium oxide. (ii) Use your diagram to interpret and explain two physical properties of magnesium oxide. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. (iii) State two large scale uses of magnesium oxide. .................................................................................................................................. .................................................................................................................................. [5] Bilal Hameed 8701/2/O/N/01 Bilal Hameed 1 2 2 21 (a) Salt, sodium chloride, forms transparent colourless crystals. Describe the bonding in sodium chloride crystals, give the formula of each particle and sketch part of the crystal structure. For Examiner’s Use [3] (b) Explain why crystals of sodium chloride3do not conduct electricity, but molten sodium chloride does. (iii) Suggest why using hydrazine as a rocket fuel could be regarded as being ‘environmentally friendly’. .......................................................................................................................................... .................................................................................................................................. .......................................................................................................................................... .................................................................................................................................. ......................................................................................................................................[2] [4] (c) (i) With the aid of a diagram of the cell, outline the manufacture of chlorine from brine (c) The bonding in sodium hydrazine is similar to that in ammonia. (aqueous chloride). (i) Showing outer-shell electrons only, draw a ‘dot-and-cross’ diagram of an ammonia molecule. (ii) Draw a diagram to show the three-dimensional shape of an ammonia molecule. (ii) Write the electrode equations, including state symbols. (iii) Draw a diagram to show the shape of a hydrazine molecule. anodeclearly ........................................................................................................................ Show which atom is joined to which and show clearly the value of one bond angle. cathode ..................................................................................................................... 9701/02/O/N/03 [4] (d) Deduce the oxidation state of nitrogen in hydrazine. Bilal Hameed .......................................... 2 [1] [Total: 12] Bilal Hameed For Examiner’s Use Examiner’s Use ......................................................................................................................................[1] oxide Na O MgO Al O SiO P O SO 2 2 3 2 3253 2503 4 10 3 33(b) Drawing diagrams where appropriate, suggest in terms of structure and bonding, melting point / K 1193 3125 2345 1883 853 290 explanations for the following. boiling point / K (i) 1548 3873 – 318 the high melting point and boiling point of Al2O3 (a) Write an equation for the reaction of aluminium with oxygen to form aluminium oxide. ......................................................................................................................................[1] (b) Drawing diagrams where appropriate, suggest in terms of structure and bonding, explanations for the following. (i) (ii) the low boiling point of SO3 (ii) (iii) the high melting point and boiling point of Al2O3 the low boiling point of SO3 the melting point of SiO2 is much higher than that of P4O10 (iii) the melting point of SiO2 is much higher than that of P4O10 [7] © UCLES 2004 9701/02/O/N/04 [7] © UCLES 2004 Bilal Hameed Bilal Hameed 9701/02/O/N/04 3 2 2 For torches’for forcutting cuttingand and welding metals. In the torch, ethyne is burned in oxygen to produce a Examiner’s torches’ welding metals. In the torch, ethyne is burned in oxygen to produce a Examiner’s Use flame with a temperature of 3400 K. Use flame with a temperature of 3400 K. 4 (a) Ethyne Ethyneisisa alinear linear molecule with a triple bond, C!C, between the carbon two carbon atoms. 44(a) molecule with a triple bond, C!C, between the two atoms. Drawaa‘dot-and-cross’ ‘dot-and-cross’ diagram of an ethyne molecule. Draw diagram of an ethyne molecule. 4 [1] 4 [1] used foriodine cutting orboth welding, ethyne ishave transported inphysical cylinders which contain the the 55(b) (b) When used cutting or welding, ethyne ishave transported inphysical cylinders which contain 22 When Copper and are solids which different and chemical properties. For Copper andfor iodine are both solids which different and chemical properties. 3 and For gas under pressure. A typical cylinder has a volume of 76 dm contains ethyne gas 3 Each element has thethe face-centred crystal structure which isand shown below.below. gas under pressure. Asame typical cylinder has acrystal volume of 76 dm contains ethyne gas Examiner’sExaminer’s Each element has same face-centred structure which is shown atat1515 kPa pressure at a temperature of 25 °C. Use 1515 kPa pressure at a temperature of 25 °C. Use Use the general gas equation, pV = nRT, to calculate the amount, in moles, of ethyne in Use the general gas equation, pV = nRT, to calculate the amount, in moles, of ethyne in this cylinder. this cylinder. [2] [2] (c) In some countries, ethyne is manufactured from calcium carbide, CaC2, which is heating quicklime coke together atfrom 2300calcium K. (c) produced In somebycountries, ethyne and is manufactured carbide, CaC2, which is produced by heating quicklime and coke together at 2300 K. CaC2be+ atoms, CO molecules, anions or cations. In the The particles present inCaO such+a3C crystal may CaO + 3C CaC CO . diagram above, present the particles present are represented by The particles in such a crystal may 2be+ atoms, molecules, anions or cations. In the When water is added to the CaC2, calcium hydroxide, Ca(OH)2, and ethyne, C2H2, are . diagram above, the particles present are represented by produced. (a) Which of particles are present in the hydroxide, iodine crystal? Give their When watertype is added to the CaC , calcium Ca(OH) , andformula. ethyne, C H , are 2 2 2 2 produced. Which type of particles are present in the iodine crystal? theircarbide. formula. (i)(a)Construct a balanced equation for the formation of ethyne fromGive calcium particle .................................... (i) Construct a balanced equation for the formation of ethyne from calcium carbide. particle .................................... .................................................................................................................................. formula .................................... [2] .................................................................................................................................. formula .................................... [2] (ii) thisseparate equation and yourofanswer (b)are to calculate CaC2 which (b) Use When samples copperto orpart iodine heated tothe 50 mass °C, theofcopper remains as will react with an excess of water to produce enough ethyne to fill 100 cylinders of a solid while the iodine turns into a vapour. (ii) Use thisseparate equationsamples and yourofanswer (b)are to calculate CaC2 which gas. (b)the When copperto orpart iodine heated tothe 50 mass °C, theofcopper remains as will reactwhile with an excess of water to produce enough ethyne to fill 100 cylinders of iodine turns intopresent a vapour. (i)a solid Explain, inthe terms of the forces in the solid structure, why copper remains a the gas. solid at 50 °C. (i) Explain, in terms of the forces present in the solid structure, why copper remains a solid at 50 °C. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. [3] .................................................................................................................................. .................................................................................................................................. (ii) (ii) [3] Explain, in terms of the forces present in the solid structure, why iodine turns into a .................................................................................................................................. vapour when heated to 50°C. Explain, in terms of the forces present in the solid structure, why iodine turns into a vapour when heated to 50°C. .................................................................................................................................. .................................................................................................................................. © UCLES 2006 © UCLES 2006 .................................................................................................................................. 9701/02/M/J/06 .................................................................................................................................. .................................................................................................................................. [4] 9701/02/M/J/06 .................................................................................................................................. [4] Bilal Hameed Bilal Hameed Bilal Hameed 4 4 Answer all the questions in the spaces provided. 61 Ethene, C2H4, and hydrazine, N2H4, are hydrides of elements which are adjacent in the Periodic Table. Data about ethene and hydrazine are given in the table below. C2H4 N2H4 melting point/°C –169 +2 boiling point/°C –104 +114 solubility in water insoluble high solubility in ethanol high high For Examiner’s Use (a) Ethene and hydrazine have a similar arrangement of atoms but differently shaped molecules. (i) What is the H-C-H bond angle in ethene? .................................................................................................................................. (ii) Draw a ‘dot-and-cross’ diagram for hydrazine. (iii) What is the H-N-H bond angle in hydrazine? .................................................................................................................................. [4] (b) The melting and boiling points of hydrazine are much higher than those of ethene. Suggest reasons for these differences in terms of the intermolecular forces each compound possesses. .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... ......................................................................................................................................[3] 3 (c) Explain, with the aid of a diagram showing lone pairs of electrons and dipoles, why hydrazine is very soluble in ethanol. © UCLES 2007 9701/02/M/J/07 [3] Ethene and hydrazine each react with HC!. Bilal Hameed (d) When ethene is reacted with HC!, C2H5C! is the only product. Bilal Hameed (i) 5 Using structural formulae, give an equation for the reaction between ethene and HC!. For Examiner’s Use For Examiner’s Use 2 Answer all the questions in the spaces provided. 77 1 This question is about the bonding of covalent compounds. (a) On the axes below, sketch the shapes of a 1s, a 2s, and a 2px orbital. z z y z y x y x 1s x 2s 2px [3] (b) Covalent bonding occurs when two atoms share a pair of electrons. Covalent bonding may also be described in terms of orbital overlap with the formation of σ bonds. (i) How are the two atoms in a covalent bond held together? In your answer, state which particles are attracted to one another and the nature of the force of attraction. .................................................................................................................................. .................................................................................................................................. (ii) Draw sketches to show orbital overlap that produces the σ bonding in the H2 and HC! molecules. H2 HC! [4] (c) The bond in the HC! molecule is said to be ‘polar’. (i) What is meant by the term bond polarity? .................................................................................................................................. (ii) Explain why the HC! molecule is polar. .................................................................................................................................. .................................................................................................................................. [2] © UCLES 2007 Bilal Hameed Bilal Hameed 9701/02/O/N/07 6 Examiner’s 2 2 Use The bonding in ethene may beofdescribed as 3 a mixture and π bonding. This (d) question concerns the chlorides the elements sodium oftoσphosphorus of the third 4 period of the Periodic Table. Each carbon atom in ethene forms three σ bonds shown below. (d) The bonding in ethene may be as a as mixture and π bonding. The points of these chlorides are given Thismelting question concerns the chlorides ofdescribed thebelow. elements sodium oftoσphosphorus of the third Use For For Examiner’s Examiner’s Use Use period of the Periodic Table. Each carbon atom chlorides in etheneare forms three σ bonds as shown below. The melting points of these given below. compound sodium chloride sodium 1081 chloride melting compound point/K magnesium aluminium silicon H H chloride chloride tetrachloride C C phosphorus(!) chloride magnesium aluminium silicon H 987 451* 203 H chloride chloride tetrachloride H H C C melting phosphorus(!) 435chloride 1081 987 451* 203 435 *sublimes H H point/K at 451 K On the diagram, sketch the π bond that is also present in ethene. [1] (a) Give the equation, with state symbols, for the reaction of phosphorus with chlorine to *sublimes at 451hydrogen K form phosphorus(!) chloride, PC!5.each burn exothermically in an excess of air. (e) Carbon, and ethene On the diagram, sketch the π bond that is also present in ethene. [1] o of phosphorus –1 (a) ......................................................................................................................................[2] Give the C(s) equation, with → state for the reaction with chlorine to + O2(g) COsymbols, (g) ∆H = –393.7 kJ mol c 2 form phosphorus(!) chloride, PC!5.each burn exothermically in an excess of air. (e) Carbon, hydrogen and ethene 88 (b) Suggest, of the structure and bonding, explanations the following. H2in (g)terms + ½O ∆H oc =for–285.9 kJ mol–1 2(g) → H2O(l) o You should draw diagrams where you think they will help your answer. ......................................................................................................................................[2] C(s) + O2(g) → CO2(g) ∆H c = –393.7 kJ mol–1 C H (g) + 3O (g) → 2CO (g) + 2H O(l) ∆H o = –1411.0 kJ mol–1 c 2 4 2 2 2 8 (b) (i) the high melting of→ sodium chloride Suggest, ofpoint the and bonding, explanations the following. H in (g)terms + ½O (g) structure H O(l) ∆H o =for–285.9 kJ mol–1 2 2 c 2 o the draw data to calculatewhere the standard enthalpy change of formation, You Use should diagrams you think they will help your answer.∆H f , in kJ mol–1, o ofC ethene at 298 K. ∆H c = –1411.0 kJ mol–1 2H4(g) + 3O2(g) → 2CO2(g) + 2H2O(l) (i) the high melting point of sodium chloride 2C(s) + 2H2(g) → C2H4(g) Use the data to calculate the standard enthalpy change of formation, ∆H of , in kJ mol–1, of ethene at 298 K. 2C(s) + 2H2(g) (ii) → C2H4(g) the low melting point of silicon tetrachloride (ii) the low melting point of silicon tetrachloride ∆H of = ................................. kJ mol–1 [3] [Total: 13] ∆H of = ................................. kJ mol–1 [3] [Total: 13] [4] [4] © UCLES © UCLES 2007 2007 9701/02/O/N/07 9701/02/O/N/07 Bilal Hameed © UCLES © UCLES 2007 2007 Bilal Hameed Bilal Hameed [Turn over 7 9701/02/O/N/07 9701/02/O/N/07 [Turn over 7 9 (iii) At low temperatures, aluminium chloride vapour has the formula A!2C!6. Draw a ‘dot-and-cross’ diagram to show the bonding in A!2C!6. Show outer electrons only. Represent the aluminium electrons by . Represent the chlorine electrons by x. [6] 103 The elements phosphorus, sulphur, and chlorine are regarded as having simple molecular structures. (a) What are the molecular formulae of each of these three elements? phosphorus ........................................ sulphur ............................................... chlorine .............................................. (b) (i) [3] Place the three elements in order of their melting points with the highest first. highest ........................................................................................................... lowest (ii) Suggest an explanation for the order you have given in (i). .................................................................................................................................. .................................................................................................................................. ..............................................................................................................................[3] Bilal Hameed (a) (i) 2 How many lone pairs of electrons are there around the oxygen atom in methoxymethane? 2 Answer all the questions in the spaces provided. 11 111 For Examiner’s For Use .................................................................................................................................. The structural formulae of water, methanol and methoxymethane, CH3OCH3, are given Examiner’s below. Use 111 The structural formulae of water, methanol and methoxymethane, CH OCH , are given Answer all the questions in the spaces provided. 3 3 Suggest below. the size of the C–O–C bond angle in methoxymethane. (ii) O O O O O O .................................................................................................................................. H H3C CH3 H H H3C [2] H H3C CH3 H H H3C (a) (i) How many lone pairs of electrons are there around the oxygen atom in (i) How many pairs ofcompound, electrons are such there around oxygen point, atom inboiling point, The physical(a) properties of alone covalent as its the melting methoxymethane? methoxymethane? vapour pressure, or solubility, are related to the strength of attractive forces between the .................................................................................................................................. molecules of that compound. .................................................................................................................................. (ii) Suggest the size of the C–O–C bond angle in methoxymethane. Suggest the size of the C–O–C bond angle in methoxymethane. These relatively(ii)weak attractive forces are called intermolecular forces. They differ in their .................................................................................................................................. strength and include the following. .................................................................................................................................. [2][2] A interactions involving permanent dipoles The physical properties of a covalent compound, B suchasasitsitsmelting melting The physical properties of a covalent compound, such vapour pressure, or solubility, are related to the strength of attractive vapour pressure, or solubility, are related to the strength of attractive molecules of that compound. interactions involving temporary or induced dipoles molecules of that compound. point, boiling point, boilingpoint, point, forces between forces betweenthethe C These relatively weak attractive forces are called intermolecular forces. They differ in their These relatively weak attractive forces are called intermolecular forces. They differ in their hydrogen bonds strength and include the following. strength and include the following. A interactions involving permanent dipoles (b) By the letters A, permanent B, or C, state the strongest intermolecular force present in each A using interactions involving dipoles of the following compounds. B interactions involving temporary or induced dipoles B each interactions involvingwrite temporary or induced For compound, the answer ondipoles the dotted line. C C hydrogen bonds hydrogen bonds ethanal (b) CH3A,CHO .............. By using the letters B, or C, state the strongest intermolecular force present in each of the following compounds. (b) By using the letters A, B, or C, state the strongest intermolecular force present in each For each compound, write OH the answer on the dotted line. ethanol CH3CH .............. of the following compounds. 2 For eachethanal compound, write the answer on3the dotted line. CH3CHO .............. methoxymethane CH3OCH3 .............. ethanal CH3CHO .............. ethanol are completely CH3CHsoluble (c) Methanol and water in.............. each other. 2OH For 3 Examiner’s 2-methylpropane (CH ) CHCH .............. [4] ethanol methoxymethane CH33CH .............. CH OCH3 3 .............. 2 23OH Use 3in each (c) intermolecular Methanol and waterforce are completely soluble other. molecules and water molecules (i) Which exists between methanol 3 For 2-methylpropane (CH )2CHCH3 in.............. .............. [4]Examiner’s thatmethoxymethane makes these twoCH liquids each other? 3OCH33soluble Use 3 methanol Which intermolecular forcesoluble existssoluble between Methanol(i) and water are in each (c) (c)Methanol and water arecompletely completely inother. eachmolecules other. and water molecules For For that makes these two liquids soluble in each other? Examiner’s 2-methylpropane (CH ) CHCH .............. [4] Examiner’s 3 2 completely 3 (c) Methanol and water are soluble in each other. .................................................................................................................................. For Use 3 methanol molecules and water molecules (i) Which intermolecular force exists between Use (i) Which intermolecular forcesoluble existsinbetween methanol molecules and waterExaminer’s molecules .................................................................................................................................. that makes these two liquids each other? Use (i) Which intermolecular force exists between methanol molecules and water molecules that these two liquids soluble each other? Methanol and water completely soluble ininintermolecular each (ii) (c) Draw amakes diagram thatare clearly shows this Yourshould diagram should For that these liquids soluble each other. other? force. force. (ii) Draw amakes diagram thattwo clearly shows thisinintermolecular Your diagram Examiner’s .................................................................................................................................. show any lone pairs dipoles present either molecule youtoconsider to show any loneorpairs or dipoles present on on either molecule that youthat consider be Usebe (i) Which intermolecular force exists between methanol molecules and water molecules .................................................................................................................................. .................................................................................................................................. important. important. that makes these two liquids soluble in each other? (ii) (ii) Draw a diagram that clearly shows this intermolecular force. Your diagram should a diagram that clearly shows intermolecular Yourconsider diagram should show (ii) any Draw lone pairs or dipoles present onthis either molecule force. that you to be Draw a diagram clearly shows thisonintermolecular Your todiagram should show anythat lone pairs or dipoles present either molecule thatforce. you consider be .................................................................................................................................. important. show any important. lone pairs or dipoles present on either molecule that you consider to be (ii) Draw a diagram that clearly shows this intermolecular force. Your diagram should important. show on either molecule that you consider to be © UCLES 2008 any lone pairs or dipoles present 9701/02/M/J/08 important. © UCLES 2008 9701/02/M/J/08 © UCLES 2008 9701/02/M/J/08 [4] (d) When equal volumes of ethoxyethane, C2H5OC2H5, and water are mixed, shaken, and 9 then allowed to stand, two layers are formed. [4] [4] Bilal Hameed [4] Suggest why ethoxyethane does not fully dissolve in water. Explain your answer. (d)volumes When equal volumes of ethoxyethane, C2OC H5OCH and water are mixed, shaken, and 2H5,, water (d) When equal ofof ethoxyethane, C 5H are shaken, mixed, shaken, and (d) When equal volumes ethoxyethane, Care OC are mixed, and 5 2H5,2and 5 and water 2H2 then allowed to stand, two layers formed. Bilal Hameed 9 Bilal Hameed 9 .......................................................................................................................................... then allowed to stand, two layers are formed. then allowed to stand, two layers are formed. [4] [4] Suggest why ethoxyethane does not fully dissolve in water. Explain your answer. .......................................................................................................................................... Suggest why ethoxyethane does not fully dissolve in water. Explain your answer. Suggest why equal ethoxyethane not fullyC2dissolve water. your answer. (d) When volumes of does ethoxyethane, H5OC2H5,in and waterExplain are mixed, shaken, and .......................................................................................................................................... (d) Whenthen equal volumes of ethoxyethane, C H OC H , and water are mixed, shaken, and .......................................................................................................................................... 2 5 2 5 allowed to stand, two layers are formed. .......................................................................................................................................... then allowed to stand, two layers are formed. .......................................................................................................................................... .......................................................................................................................................... ......................................................................................................................................[2] Suggest why ethoxyethane does not fully dissolve in water. Explain your answer. .......................................................................................................................................... .......................................................................................................................................... Suggest why ethoxyethane does not fully dissolve in water. Explain[Total: your12]answer. Bilal.......................................................................................................................................... Hameed .......................................................................................................................................... .......................................................................................................................................... ......................................................................................................................................[2] .......................................................................................................................................... .......................................................................................................................................... 44 44 12 122 Ketene, 12 Ketene, CO, His O,a is is member of class of unsaturated unsaturated aa member aa class of organic compounds is widely For Ketene, C2H HC member of aa of class of unsaturated unsaturated organic compounds that that is widely 22His 22O, For 22 2Ketene, C a member of class of 2O, For 2in 2 For used pharmaceutical research for the synthesis of organic compounds. Examiner’s used in pharmaceutical research for the synthesis of Examiner’s used in pharmaceutical research for the synthesis of organic compounds. Examiner’s used in pharmaceutical research for the synthesis of organic Examiner’s Use Use Use Use CH2=C=O =C=O CH CH2=C=O =C=O 2 CH 2 ketene ketene ketene ketene (a) (i) Suggest Suggest values forH-C-H the H-C-H H-C-H and C=C=O C=C=O bond (i) values for the and angles in ketene. (a) (a) (i) Suggest Suggest values for the the and C=C=O C=C=O bondbond angles in ketene. (a) (i) values for H-C-H and bond H-C-H …………………………………… C=C=O …………………………………… H-C-H …………………………………… C=C=O H-C-H …………………………………… C=C=O …………………………………… H-C-H …………………………………… C=C=O (ii) By considering considering the structure structure of molecule, the molecule, molecule, suggest why the name ketene is By the of the (ii) (ii) By considering considering the structure structure of the the (ii) By the of molecule, suggest why the name ketene is used. used. used. used. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. 22 ............................................................................................................................. [3] ............................................................................................................................. ............................................................................................................................. [3] [3] [3] ............................................................................................................................. Answer all the questions in the spaces provided. Answer all the questions in the spaces provided. (b) Ketene burns completely intoair air to form form carbon dioxide and water. water. (b) Ketene burns completely to carbon dioxide and (b) Ketene burns completely in air airin form carbon dioxide and water. water. (b) Ketene burns completely in to are form carbon dioxide and 13 13 1 The elements carbon and silicon both in Group IV of the Periodic Table. 131 The elements carbon and silicon are both in Group IV of the Periodic Table. (i) Write a balanced equation for this reaction. Carbon is the second most abundant element by mass in the human body and and silicon silicon is is the the (i) a balanced equation forelement this reaction. Carbon isWrite the second most abundant by mass in the human body (i) Write Write a balanced balanced equation for this this reaction. (i) a equation for reaction. second most most common common element element in in the the Earth’s Earth’s crust. crust. second .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. Carbon and silicon silicon each each form form an an oxide oxide of of general general formula formula XO XO2.. Carbon and 2 33,, measured (ii) Use your equation to calculate the volume of CO ,high in33 dm dm measured at room room At room temperature, CO is a gas while SiO is a solid with a2,dm high melting point. (ii) Use your equation to calculate the volume of CO in at At room temperature, CO is a gas while SiO is a solid with a point. 2 2 (ii) Use your equation to calculate the volume of CO , in , melting measured at room room 2dm 2 2 (ii) Use temperature your equation to calculate the volume of CO , in , measured at 2 2 and pressure, which will be formed when 3.5 g of ketene are burned in temperature and pressure, which willformed be formed g of ketene are burned temperature and pressure, pressure, which will be be whenwhen 3.5 gg3.5 of ketene ketene are burned burned in in temperature and which will formed when 3.5 of are in an excess of air. (a) Briefly explain, in terms of the chemical bonds and intermolecular forces present in each anexplain, excess of terms air. of the chemical bonds and intermolecular forces present in each (a) Briefly in an excess excess of air. air. an of compound, why CO CO2 is is aa gas gas and and SiO SiO2 is is aa solid solid at room temperature. compound, why 2 to 2 figures. Give your your answer answer to two two significant significant figures. at room temperature. Give Give your your answer answer to to two two significant significant figures. figures. Give .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... .................................................................................................................................... [3] [3] .................................................................................................................................... (b) Draw Draw aa simple simple diagram diagram to to show show the the structure structure of of SiO SiO2.. Your Your diagram diagram should should contain contain at at (b) 2 least two silicon atoms and show clearly how many bonds each atom forms. least two silicon atoms and show clearly volume how many bonds each atom forms. dm33 [4] of CO CO = ................................. volume of volume of CO CO = ................................. ................................. dm33 dm [4] [4] 22 = ................................. volume of = dm [4] 2 2 UCLES 2008 2008 ©© UCLES UCLES 2008 ©©UCLES 2008 Bilal Hameed Hameed Bilal Bilal Hameed 9701/02/O/N/08 9701/02/O/N/08 9701/02/O/N/08 9701/02/O/N/08 [2] [2] 10 10 For For Examiner’s Examiner’s Use Use Answer all the questions in the spaces provided. 141 14 For Examiner’s Use Elements and compounds which have small molecules usually exist as gases or liquids. (a) Chlorine, C!"2, is a gas at room temperature whereas bromine, Br 2, is a liquid under the same conditions. Explain these observations. .......................................................................................................................................... .......................................................................................................................................... .................................................................................................................................... [2] (b) The gases nitrogen, N2, and carbon monoxide, CO, are isoelectronic, that is they have the same number of electrons in their molecules. Suggest why N2 has a lower boiling point than CO. .......................................................................................................................................... .......................................................................................................................................... .................................................................................................................................... [2] (c) A ‘dot-and-cross’ diagram of a CO molecule is shown below. Only electrons from outer shells are represented. C O In the table below, there are three copies of this structure. On the structures, draw a circle round a pair of electrons that is associated with each of the following. (i) a co-ordinate bond C O (ii) a covalent bond C O (iii) a lone pair C O [3] © UCLES 2010 Bilal Hameed Bilal Hameed 9701/21/M/J/10 11 4 15 15 2 Copper, proton number 29, and argon, proton number 18, are elements which have different physical and chemical properties. In the solid state, each element has the same face-centred cubic crystal structure which is shown below. The particles present in such a crystal may be atoms, molecules, anions or cations. In the diagram above, the particles present are represented by . (a) Which types of particle are present in the copper and argon crystals? In each case, give their formula. element particle formula copper argon [2] At room temperature, copper is a solid while argon is a gas. (b) Explain these observations in terms of the forces present in each solid structure. .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... .................................................................................................................................... [4] © UCLES 2010 Bilal Hameed Bilal Hameed 9701/22/M/J/10 12 For Examiner’s Use Although copper is a relatively unreactive element, when it is heated to a high temperature in 5 an excess of chlorine, copper(!!) chloride is formed. Although copper is a relatively unreactive element, when it is heated to a high temperature in For Examiner’s Use For When a mixture of argon and chlorine an excess of chlorine, copper(!!) chloride is is heated formed. to a high temperature, no reaction occurs. Examiner’s Use When of argon and chlorine to a high temperature, (c) (i)a mixture How does chlorine behaveisinheated its reaction with copper? no reaction occurs. (c) (i) How does chlorine behave in its reaction with copper? .................................................................................................................................. (ii) .................................................................................................................................. Suggest a reason for the lack of a reaction between argon and chlorine. (ii) Suggest a reason for the lack of a reaction between argon and chlorine. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. [2] .................................................................................................................................. [2] The melting points of the noble gases neon to xenon are given below. The melting points of the noble gases neon to xenon are given below. Ne melting point /K melting point /K 25 Ne 25 Ar Ar 84 Kr 84 116 Kr Xe 116 161 Xe 161 (d) Explain why there is an increase in melting point from neon to xenon. (d) Explain why there is an increase in melting point from neon to xenon. .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... .................................................................................................................................... [2] .................................................................................................................................... [2] 7 7 [Total: 10] [Total: 10] 16(d) Separate samples of the oxides MgO and SiO are melted. 16 16 2 (d) Separate samples of the oxides MgO and SiO 2 are melted. For For Examiner’s Examiner’s Use Each molten sample is then tested to see whether or not it conducts electricity. Each molten sample is then tested to see whether or not it conducts electricity. Use Suggest what would be the results in each case. Explain your answers. Suggest what would be the results in each case. Explain your answers. MgO ................................................................................................................................. MgO ................................................................................................................................. .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... SiO2 ................................................................................................................................. SiO2 ................................................................................................................................. .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... [4] .......................................................................................................................................... [4] [Total: 12] [Total: 12] © UCLES 2010 © UCLES 2010 Bilal Hameed Bilal Hameed 9701/22/M/J/10 [Turn over [Turn over 9701/22/M/J/10 13 17 172 172 2 2 4 4 Carbon disulphide, CS2, is a volatile, stinking liquid which is used to manufacture viscose Carbon disulphide, CS , is a volatile, stinking liquid which is used to manufacture viscose rayon and cellophane. 2 rayon and cellophane. 4 4 (a) The carbon atom is in the centre of the CS2 molecule. (a) Theand carbon atom is in the CS2 molecule. Sulphur its compounds arecentre found of in the volcanoes, in organic matter and in minerals. Sulphur and its compounds are found in volcanoes, in organic matter and in minerals. Draw a ‘dot-and-cross’ diagram of the carbon disulphide molecule. Draw acid, a ‘dot-and-cross’ diagram of thechemical, carbon disulphide molecule.from sulphur by the Sulphuric an important industrial is manufactured Sulphuric acid, an important industrial chemical, is manufactured from sulphur by the Contact process. There are three consecutive reactions in the Contact process which are Show outer electrons only. Contact process. There are three consecutive reactions in the Contact process which are Show outer electrons only. essential. essential. (a) Write a balanced equation (using (a) Write a balanced equation (using in the correct sequence. in the correct sequence. where appropriate) for each of these reactions where appropriate) for each of these reactions 1 ....................................................................................................................................... 1 ....................................................................................................................................... [2] [2] 2 ....................................................................................................................................... 2 ....................................................................................................................................... (b) Suggest the shape of the molecule and give its bond angle. (b) 3Suggest the shape of the molecule and give its bond angle. ................................................................................................................................. [4] 3 ................................................................................................................................. [4] shape ......................................................... shapecatalyst ......................................................... (b) What is used? (b) What catalyst is used? bond angle ................................................. [2] bond angle ................................................. [2] .................................................................................................................................... [1] .................................................................................................................................... [1] (c) Explain the term standard enthalpy change of formation, !H f . (c) Explain the termHstandard enthalpy change of formation, Hydrogen sulphide, S, is a foul-smelling compound found !inHthe gases from volcanoes. f . gases Hydrogen sulphide, H22S, is a foul-smelling compound found in the from volcanoes. Hydrogen sulphide is covalent, melting at –85 °C and boiling at –60 °C. Hydrogen sulphide is covalent, melting at –85 °C and boiling at –60 °C. .......................................................................................................................................... .......................................................................................................................................... (c) (i) to show show the the structure structure of of the the H H2S S molecule. (c) .......................................................................................................................................... (i) Draw Draw aa ‘dot-and-cross’ ‘dot-and-cross’ diagram diagram to 2 molecule. .......................................................................................................................................... .................................................................................................................................... [3] .................................................................................................................................... [3] (d) of formation formation of of CS CS2 from from the the following following data. data. (d) Calculate Calculate the the standard standard enthalpy enthalpy change change of 2 standard of SO SO2 standard enthalpy enthalpy change change of of formation formation of 2 –1 = –298 –298 kJ kJ mol mol–1 = standard of CO CO2 standard enthalpy enthalpy change change of of formation formation of 2 (ii) Predict the shape of the H S molecule. (ii) Predict the shape of the H22S molecule. standard of CS CS2 standard enthalpy enthalpy change change of of combustion combustion of 2 ............................................................. ............................................................. –1 = –395 –395 kJ kJ mol mol–1 = –1 = –1110 –1110 kJ kJ mol mol–1 = (iii) Group VI VI of of the the Periodic Periodic Table. Table. (iii) Oxygen Oxygen and and sulphur sulphur are are both both in in Group Suggest points of of water, water, H H2O, O, are are much much higher higher than than Suggest why why the the melting melting and and boiling boiling points 2 those of H S. those of H2 S. 2 .................................................................................................................................. .................................................................................................................................. [3] .................................................................................................................................. [3] .................................................................................................................................. ............................................................................................................................ [4] ............................................................................................................................ [4] © © UCLES UCLES 2005 2005 9701/02/O/N/05 9701/02/O/N/05 Bilal Hameed © Bilal © UCLES UCLES 2005 2005 Bilal Hameed Hameed 9701/02/M/J/05 9701/02/M/J/05 14 14 For For Examiner’s Examiner’s Use Use For For Examiner’s Examiner’s Use Use