CLINICAL TRIAL FINANCIAL PROCESS RESEARCH TEAM SELF
advertisement

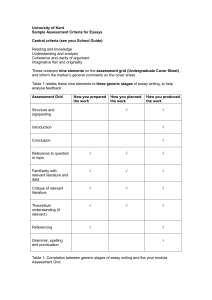
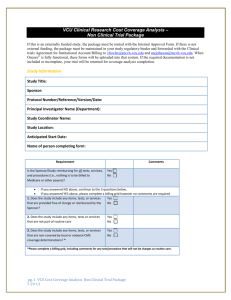
CLINICAL TRIAL FINANCIAL PROCESS RESEARCH TEAM SELF-ASSESSMENT WORKSHEET Study Name___________________________________________________________________________________________________________ IRB Number_____________ Date Review Completed___________________________ Principal Investigator_______________________________________ Billing Grid? YES NO (circle one) Budget Worksheet YES Reviewed By:_______________________________ Study Coordinator___________________________________________ NO Number of Subjects enrolled____________________________ Budget/ Billing Grid Review Issue Comments Y / N / NA 1. Is the trial for an IDE device? 2. If not an IDE trial, does the trial qualify under Medicare NCD? (check for all four criteria) 3. If IDE, was permission obtained from the MC Contractor? (not required if no MC subjects to be enrolled) 4. Does the CTA have a detailed budget? 5. If the CTA does not have a detailed budget, does the study team have one on file? 6. Does the research team have a billing grid? If no to grid questions, create or amend the grid 7. General nature of funding? Is Sponsor paying for all? Billing to Insurance? Is there a mixture of both? 8. Does the grid include all clinical services required by the Protocol? 9. Does the grid clearly define what is Sponsor paid? 10. Is the Grid consistent with the budget? 11. Is the grid consistent with MC billing guidelines? 12. Is Sponsor paying for unbillable items/services 13. Does trial need an NCT # Place here if yes Informed Consent Form Review (ICF) 14. Is the research-related injury paragraph consistent with If not, correct ICF and resubmit to IRB the CTA? 15. Does the cost section clearly identify what the patient must pay for? “ 16. Is the cost section consistent with the budget? “ Either ICF or Grid must be corrected 17. Is the cost section consistent with the billing grid? 18. Are any services promised as free in the ICF which are legally billable? (not being paid by sponsor) If yes, correct ICF and resubmit to IRB 9if subjects were billed, research team must request refund to subject/insurer and bill research accounts Verification of Bills Received by Research Team 19. Have any participants been enrolled in the study and received study items or services? 20. Have the consented subjects been identified and flagged appropriately for billing purposes? 21. Does the research team/site have a process for verifying all bills received for all participants (sponsor-funded) If not, develop a process (SOP) 22. For participants who received sponsor-funded services more than 60 days prior to this review, is there documentation (ledger, bills, etc.) that the team received the bill? Tracking and Reconciling Sponsor Payments Obligated by the Study Contract 23. Is there a system in place to track the study payments If not, develop a process (SOP) owed by the sponsor? 24. Is there an invoicing process and an identifiable invoice tracking method to ensure that all earned payments are received? “ 25. Are sponsor payments being reconciled with what is owed by the sponsor? “ Additional Comments: V1 9/15/2014