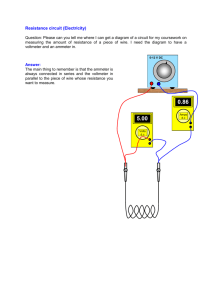
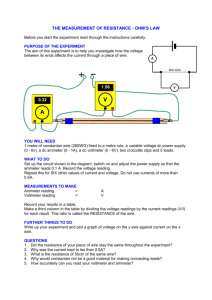
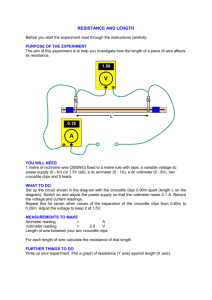
55-80 - Delhi
advertisement
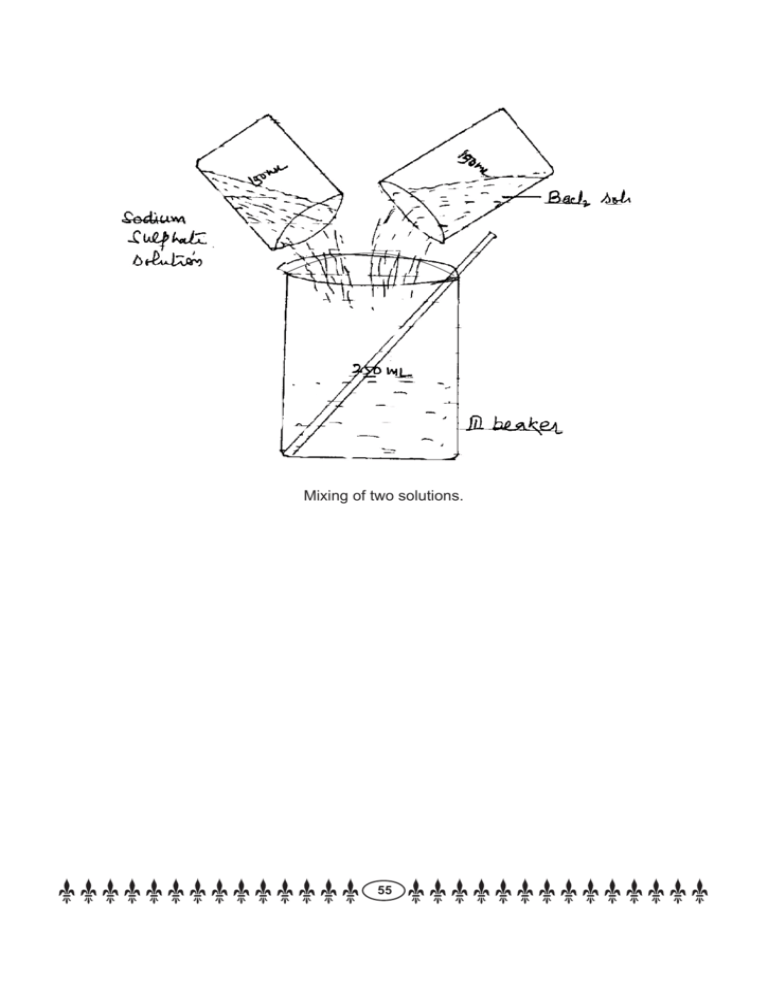
Mixing of two solutions. 55 Class IX EXPERIMENT No: 9 AIM: To study the external features of root, stem, leaf and flower of monocot and dicot plants. Material Required : Plants of Hibiscus/Peturnia/rose/pea and grass/maize/bamboo/lily or, any other ornamental herb with flower and fruits, simple or dissecting microscope, hand lens, slide, coverslip and razor /blade. Procedure : 1. Observe the differences in the external features of stem, leaf, root, flowers and seeds. 2. To study the leaf, see their shape and venation i.e. parallel or reticulate. 3. To study the roots, wash them properly and then spread on the paper and study their nature i.e. tap root or fibrous. 56 4. To study the flower, count the number of sepals & petals in the flower is trimerous or pentamerous. 5. Remove the seed coat and count the number of cotyledons is mono cotyledon or dicotyledon. 6. Take a transverse section of ovary and count the number of carpels ie bicarpillary or tricarpellary. 57 Observations : Observe the important features that distinguish a monoct and a dicot plant and list the features of difference between than in the table given below. S. No. Feature Monocot 1. Leaf venation: (Parallel/Reticulate) 2. Leaf shape : (broad/narrow) 3. Roots : Fibrous/ tap root 4. Floral parts : multiple of 3 or 5 5. Sepal : number and colour 6. Petals : number and colour 7. Pistil : number of carpels 8. Cotyledon : one or two Dicot Inference : The monocot plants can be differentiated from dicot plants by the presence of parallel venation in leaf, narrow leaves, fibrous roots, floral parts in the multiple of 3 and one cotyledon in their seeds. Precautions : 1. A hand lens must be used to see the leaf venation or number of cotyledons. 2. Small / Tiny plants must be selected for study. 3. The plants bearing plowers and/or seed must be used tor studying. 58 Class IX EXPERIMENT No: 10 AIM: To study the life cycle of mosquito. Materials Required : Charts showing the life cycle of a mosquito and/or museum specimen of stages in the life cycle. Permanent slides and compound microscope. Procedure : 1. Observe the preserved specimen and name the stages. 2. Observe the chart carefully and note the different stages in the life cycle. 3. Discuss the characterstics of each stage. Observations : Eggs : Deposited in water, they hatches in water. Larva : Lives on the surface of water, moults several time. Pupa : A stage just prior to the adult stage, pupae do not feed. Adult : It emerges from pupae body parts harden and starts flying. Precautions : 1. The chart should clearly indicate the life stages of mosquito. 2. The hand lens should be used to see the different stages of specimen. 59 Class X EXPERIMENT No: 1 AIM: To find pH of the following samples using pH paper a. Dil. Hcl b. Dil. solution of NaOH c. Dil. solution of acetic acid d. Lemon juice e. Tap water f. Dil. solution of Na2 CO3 Materials Required : All above solution samples, tap water, pH paper with standard colour chart, dropper and white tile. Procedure : 1. Take above mentioned samples separately and clean test tubes labelled as (a) to (f) respectively. 3. Take separate strip of pH paper and place it on a clean and dry white glazed tile corers pending to each sample. 4. Compare the colour produced on pH paper with the standard colour chart & record the corresponding value of pH. Observations : Sample a Dil. Hcl b NaOH C Acetic Acid d Lemon Juice e Tap Water f Na2 CO3 pH 60 Inference : a. When pH is 7, neutral b. When pH is above 7, sample is basic c. When pH is below 7, smaple is acidic We find that HCl, Acetic acid and lemon juice are acidic while NaO2 and Na2 Co3 are basic in nature Tap water is .................... Precautions : 1. pH paper should not be touched with wet hand or dirty hand. 2. Separate dropper should be used for each sample. 3. Use colour chart given with the corresponding pack of pH paper. (Don’t use colour chart of any other packet. 61 Class X EXPERIMENT No: 2 AIM: (a) To study the properties of on acid it’s reaction with (i) Litmus (ii) Zine (iii) Na2 Co3 (b) To study the properties of a base NaOH by it’s reaction with (i) Litmus (ii) Zine (iii) Hcl Part (a) Materials Required : Hydrochloric acid, blue Litmus solution, Zinc granules, Na2 Co3 dropper and test tubes. Observation Table : Experiment Observation Inference 1. Take 1 ml dil. HCl in a test tube and add a few drops of blue litmus solution. Solution turns red HCl has acidic nature 2. Take some zinc granules in a test tube and 1 ml of dil. HCl to it. Bubbles of H2 gas are given out HCl reacts with Zn to form H2 gas Brick effervesence is produced due to the HCl and Na2 CO3 react to give CO2 gas 3. a. Take 1 ml dil. HCl in a test and add a pinch of solid b. Na2 CO3 to it. founation of CO2 gas Pass the gas through freshly prepared lime water Lime water turns milky. Resykt : Hydrochloric acid i. Shows acidic nature ii. turn blue Litmus red iii. reacts with zinc to give hydrogen gas iv. reacts with Na2 CO3 to give CO2 gas. Precautions : Use clean test tube for litmus test part (b) Materials Required : Dil. Hcl, NaOH solutions (aq.), Test tubes, red litmus solution, zinc granules and dropper etc. 62 Observation Table : Experiment Observation Inference 1. Take 1 ml NaOH (aq.) in a test tube and add a few drops of red litmus solution. Solution turns blue NaOH is basic in nature. 2. Take 2 ml NaOH (aq.) in a clean test tube and add 2 pieces of Zn granule to it. Bubbles of H2 gas are given out. Zn reacts with NaOH solution to give H2 gas 3. Take 3 ml Na OH (aq.) in a clean test tube and two drops of phenolphalein solution to it. Pink coloration is produced NaOH is a base 4. Add dil. Hcl to above solution drop by drop Solution be comes colourless HCl nentralizes NaOH Inference : Sodium hydroxide i. show basic nature ii. turns red litmus blue iii. reacts with Zn to give H2 gas iv. is neutralized by HCl Precaution : 1. Use NaOH carefully. It is corossive 2. Use clean test tube for phenolphthalein test or litmus test. 63 Class X EXPERIMENT No: 3 AIM: To perform and observe the following reactions and classify them into : 1. i. Combination reaction. ii. Decompositions reactions. iii. Displacement reaction. iv. Double displacement reaction. 1. Action of water on quick lime. 2. Action of heat on ferrous sulphate. 3. Iron nails kept in copper sulphate solution. 4. Reaction between sodium sulphate and barium chloride solution. Action of water on quick lime Materials Required : Quick lime, distilled water beaker, test tubes, filtrations set, red litmus paper. Procedure : Perform the action of water on quick lime according to the following table : Experiment Observation Inference 1. Take 40 ml of water in a beaker and add 4 g of quick lime to it. Stir it with glass rod. Brisk effervescence begins. A new substance in formed, 2. Touch outer surface of the beaker It is hot Heat in evolved during the reaction. 3. Place a drop of liquid of liquid of beaker on a red litmus paper. It turns blue New substance formed in pasic in nature 4. Take about 10 ml filtered liquid from the above beaker and blow air from mother. It turns milky New substance formed is slaked lime Result : 1. Water and quick lime directly combine to from calcium by hydroxide along with li beration of heat. 2. The reaction is : (i) Combination reaction 64 (ii) Exothermic reaction Precautions : \2. 1. Quick lime should not be touched with hands. 2. Add quick lime very carefully to avoid spurting of the mixture. Action of heat on ferrous sulphate : Materials Required : Ferrous sulphate crystals (Solid), Acidified potassium dichromates solution, blue litmus paper, test tubes, test tube holder. Procedure : Experiment Observation Inference 1. 2 g of solid ferrous sulphate is taken in a clean dry test tube and heat it. A colorless gas forms leaving a brown yellow solid in test tube. Ferrous sulphate decompose on heating 2. Smell the gas carefully. It smells like burning sulphur. The gases are oxides of sulphur. 3. Bring a moist blue litmus paper near the mouth of test tube. It turns to red. Oxides of sulphur are acidic in nature. Result : 1. Ferrous sulphate on heating decomposes and produce ferric acids & oxides of ferrous. 2. The type of reaction in thermal Decomposition reaction. Precautions : 3. 1. The test tube should well cleaned & dry. 2. Smell the gas carefully. 3. Mouth of test tube should be kept away from you while neating. Iron nail is kept in Copper Sulphate Solution : Materials required : Copper sulphate solution, iron nails, test tube, thread, stand. Procedure : 1. Experiment Observation Inference Add 2 g copper sulphate in 20 ml of water in a test tube and place 2 iron nails. After few minutes a brown coating forms on iron nail. Copper is displaced by iron from its solution. 65 2. Compare the colour of iron nail and solutions before & after the experiment is done. Iron nail colour turns brown where as the solution turns light green. Iron is more reactive them copper. Result : 1. Iron being more reactive, it displaces copper form the solution. 2. The type of reaction is displacement reaction.. Precautions : 1. Iron nail should be cleaned before dipping in solution. 2. Reaction between sodium sulphate and barium chloride. Materials required : Solid sodium sulphate, barium chloride, distilled water, conical black, test tubes. Procedure : Experiment Observation Inference 1. Take 10 ml solution of sodium sulphate in a test tube and 10 ml solution of barium chloride in another test Tube. Mix together in a beaker. A white precipitate is observed immediately. White precipitate is barium sulphate. 2. The conical precipitate is left undisturbed for some time. It settles down in the bottom of flosk. Barium sulphate is insoluble in water. Ruesult : 1. On mixing the solution of sodium sulphate and barium chloride a white precipitate is obtained. 2. The type solutions reaction is Double displacement reaction. Precautions : 1. The solutions should be prepared in distilled water. 2. Use the chemicals in small quantity. 66 Class X EXPERIMENT No: 4 AIM: a. To observe action of Zn, Fe, Cu and Al metals on the following salt solutions (aq.) 1. Zn So4 2. Fe So4 3. Cu So4 4. Al2 (SO4)3 b. To arrange Zn, Fe, Cu and Al metals in the decreasing order of reactivity. Materials Required : Test Tubes, Test tube stands, Beaker, small pieces of metals- Cu, Al, Zn and Fe. Saturated aq. Solution of Zn So4, FeSO4, CuSO4 and Al2 (SO4)3. Procedure : 1. Take 10 ml aq. Solution of Al2(SO4)3, FeSO4 and CuSO4 in 3 test tubes separately. Add 2 small pieces of Zn granules in each. 2. Take 10 ml of aq. Solution of Al2 (SO4)3, ZnSO4 and FeSO4 in 3 test tubes separately. Add 2 pieces of copper in each. 3. Take 10 ml of aq. Solution of ZnSO4 FeSO4 and CuSO4 in 3 test tubes separately. Add 2 small pieces of Al in each. 4. Take 10 ml of aq. solution of Al2 (SO4)3, ZnSO4 and CuSO4 in 3 test tubes separately. Add 2 small pieces of Fe in each. Observation for Zinc metal : Solution Al2(SO4)3 FeSO4 CuSO4 Observation No Reaction Fe is displaced Cu is displaced Observation for Copper metal : Solution Al2(SO4)3 ZnSO4 FeSO4 Observation No reaction No Reaction No Reaction Solution ZnSO4 FeSO4 CiSO4 Observation Zn is displaced Fe is displaced Cu is dispalced Observation for Al metal : 67 Observation for Fe metal : Solution ZnSO4 CuSO4 Al2(SO4)3 Observation No reaction Cu is No reaction. Result : On the basis of above expt. Reactivity of given metals is Al > Zn > Fe > Cu Precautions : 1. Test tubes should be cleaned properly before taking any solution. 2. Test tubes should be labelled properly to identify the solution taken in the respective test tube. 68 Class X EXPERIMENT No: 5 AIM: To study the dependence of potential difference (v) across a resistor on the current (i), and determine its resistance. Also plot a graph between v and i. APPARATUS/ MATERIALS REQUIRED : 4 dry cells ( 1.5 V each ) or a battery eliminator, copper wires with bare ends, one way key, an ammeter, a voltmeter, a rheostat, a resistor ( approximately 5 ohm ), sand paper 69 PROCEDURE: 1. Rub the ends of the connecting wires with sand paper, so that the shining copper metal is seen. 2. Remove the plug from the one way key, K. 3. Connect the key, the rheostat, the ammeter, the voltmeter, cells or battery eliminator and the resistor as shown in the diagram. 4. Insert the plug key and check the deflection in the ammeter and voltmeter. 5. Adjust the slider of the rheostat in such a manner that the current passing through the resistor is small ( about 0.1 A ). 6. Read the value of the potential difference from the voltmeter. 7. Record the value of current and potential difference and take out the plug key. 8. Repeat the experiment 5 to 6 times for different values of current in ammeter and record the potential difference from the voltmeter. OBSERVATION: Least count of ammeter =………. Least count of voltmeter =………. S. No. Current (A) Potential difference (V) Resistance R= V / I in ohm 1 2 3 4 5 6 Mean Resistance = (R1 + R2 + R3 + R4 + R5 + R6) / 6 = …….. ohm Plot the graph between (V) and (I) by taking I on the x axis and V on the y axis. Join the points. The graph obtained is a straight line. RESULT: 1. The resistance of the conductor is the ratio of the potential difference and the current. 70 2. The graph is a straight line suggesting V is directly proportional to I. This verifies the ohm's law. 3. The slope of the graph V / I is the magnitude of the resistance. PRECAUTIONS: 1. Clean the ends of the connecting wires by sand paper. 2. Connections should be tight. 3. The plug key should be inserted only when you are ready to record the reading. 4. Remove the plug key when you have finished your reading. 5. Keep the value of current in the circuit as low as possible. 6. Zero errors of the voltmeter and ammeter should be taken into account and adjusted accordingly. 71 Class X EXPERIMENT No: 6 AIM: To determine the equivalent resistance of two resistors connected in series. APPARATUS / MATERIALS REQUIRED: 4 dry cells ( 1.5 V each ) or a battery eliminator, copper wires with bare ends, one way key, an ammeter, a voltmeter, a rheostat, two known resistors ( approximately 1 to 5 ohm ), sand paper 72 PROCEDURE: 1. Rub the ends of the connecting wires with sand paper, so that the shining copper metal is seen 2. Remove the plug from the one way key, K. 3. Connect the key, the rheostat, the ammeter, the voltmeter, cells or battery eliminator and the two resistors in series as shown in the diagram. 4. Insert the plug key and check the deflection in the ammeter and voltmeter. 5. Adjust the slider of the rheostat in such a manner that the current passing through the resistors is small. 6. Read the value of the potential difference from the voltmeter. 7. Record the value of current and potential difference and take out the plug key. 8. Repeat the experiment 5 to 6 times for different values of current in ammeter and record the potential difference from the voltmeter. OBSERVATION: Least count of ammeter =………. Least count of voltmeter =………. S. No. Current (A) Potential difference (V) Resistance R= V / I in ohm 1 2 3 4 5 6 Mean Resistance = (R1 + R2 + R3 + R4 + R5 + R6) / 6 = …….. ohm RESULT: The mean resistance of the resistors is equal to the sum total of the individual resistances of the resistors and is according to the formula Rs = R1 +R2 PRECAUTIONS: 1. Clean the ends of the connecting wires by sand paper. 73 2. Connections should be tight. 3. The plug key should be inserted only when you are ready to record the reading. 4. Remove the plug key when you have finished your reading. 5. Keep the value of current in the circuit as low as possible. 6. Zero errors of the voltmeter and ammeter should be taken into account and adjusted accordingly. 74 Class X EXPERIMENT No: 7 AIM: To determine the equivalent resistance of two resistances when connected in parallel. APPARATUS / MATERIALS REQUIRED: 4 dry cells ( 1.5 V each ) or a battery eliminator, copper wires with bare ends, one way key, an ammeter, a voltmeter, a rheostat, two known resistors ( approximately 1 to 5 ohm ), sand paper. 75 PROCEDURE: 1. Rub the ends of the connecting wires with sand paper, so that the shining copper metal is seen. 2. Remove the plug from the one way key, K. 3. Connect the key, the rheostat, the ammeter, the voltmeter, cells or battery eliminator and the two resistors in parallel as shown in the diagram. 4. Insert the plug key and check the deflection in the ammeter and voltmeter. 5. Adjust the slider of the rheostat in such a manner that the current passing through the resistors is small 6. Read the value of the potential difference from the voltmeter. 7. Record the value of current and potential difference and take out the plug key. 8. Repeat the experiment 5 to 6 times for different values of current in ammeter and record the potential difference from the voltmeter. OBSERVATION: Least count of ammeter =………. Least count of voltmeter =………. S. No. Current (A) Potential difference (V) Resistance R= V / I in ohm 1 2 3 4 5 6 Mean Resistance = (R1 + R2 + R3 + R4 + R5 + R6) / 6 = …….. ohm RESULT: The mean resistance of the resistances in parallel is less than the individual resistors and is according to the formula Rp = R1R2 / (R1+R2) PRECAUTIONS: 1. Clean the ends of the connecting wires by sand paper. 76 2. Connections should be tight. 3. The plug key should be inserted only when you are ready to record the reading. 4. Remove the plug key when you have finished your reading. 5. Keep the value of current in the circuit as low as possible. 6. Zero errors of the voltmeter and ammeter should be taken into account and adjusted accordingly. 76 Class X EXPERIMENT No: 8 AIM: To prepare a temporary mount of a leaf peel to show stomata. MATERIAL REQUIRED: Fresh leaves of a plant such as Petunia, Dianthus or Lily, watch glass,slide,cover slip,brush,a piece of blotting paper, razor blade ,safranin,glycerine,compound microscope. PROCEDURE: 1. Remove a peel from the lower surface of the leaf as it has more stomata as compared to the upper surface of leaf. This can be done by carefully pulling out a thin peel with the help of a razor/blade. 2. Immediately put the leaf peel in water in watch glass. 3. Now add a few drops of safranin stain to the watch glass to stain the leaf peel. 4. Cut a small piece of leaf peel. 5. On a slide put a drop of glycerin in the centre. Now place this leaf peel on the slide. 6. Gently place the cover slip. 7. Remove the excess stain and glycerin with the help of the blotting paper. 8. Now observe the stomata under the compound microscope under low power. (10X) OBSERVATION: Observe the stomata under the microscope field. The shape of Guard cell, the number of chloroplasts in the guard cells should be observed. 77 Inference: A stoma is a pore, found in the leaf and stem epidermis that is used for gaseous exchange. The pore is bordered by a pair of specialized cells known as guard cells that are responsible for regulating the size of the opening. The term stomata is also used collectively to refer to an entire stomatal complex, both the pore itself and its accompanying guard cells.The guard cell are responsible for opening of stoma. These have a number of chloroplasts. When the guard cells are shrunk the stoma closes! Precautions: 1. Do not use excess stain while preparing the slide. 2. Mount the peel in the centre of slide. 3. Avoid air bubbles while putting the cover slip. 4. Adjust the microscope properly to see the stomata. 78 Class X EXPERIMENT No: 9 AIM: To show experimentally that light is necessary for photosynthesis. Materials Required : Potted plant, black paper strip, test tube, alcohol/ethanol, water petri dish, iodine solution. Procedure : 1. Keep potted plant in dark for about two days. 2. Fix black paper stip on a leaf as shown in the figure. 3. Put the plant in sunlight for about eight hours. 4. Pluck leaf, remove black paper strip. 5. Boil the leaf in alcohol/ethanol using water bath. 6. Wash this leaf in hot water to make it soft. 7. Place leaf in petri dish and pour 2 or 3 iodine drops. Light is necessary for Photosynthesis Observations : 1. During boiling, green leaf looses colour and become pale yellow. 2. Water treatment to leaf makes it soft. 3. After iodine treatment leaf portion not covered by black paper stip changed to dark blue colour. 79 4. Black paper covered area does not show positive result to starch iodine test. Inference : 1. Black paper strip does not allow light to fall on covered portion. 2. Photosynthesis takes place in the area exposed to light. 3. Photosynthesis does not take place in black paper strip covered portion. 4. It indicates that light is necessary for photosynthesis. Precautions : 1. Potted plant should be completely destarched in the beginning. 2. Black paper strip should not allow light to enter covered leaf area. 3. Always use water bath to boil leaf in alcohol/ethanol. 80