Engineering Data
advertisement
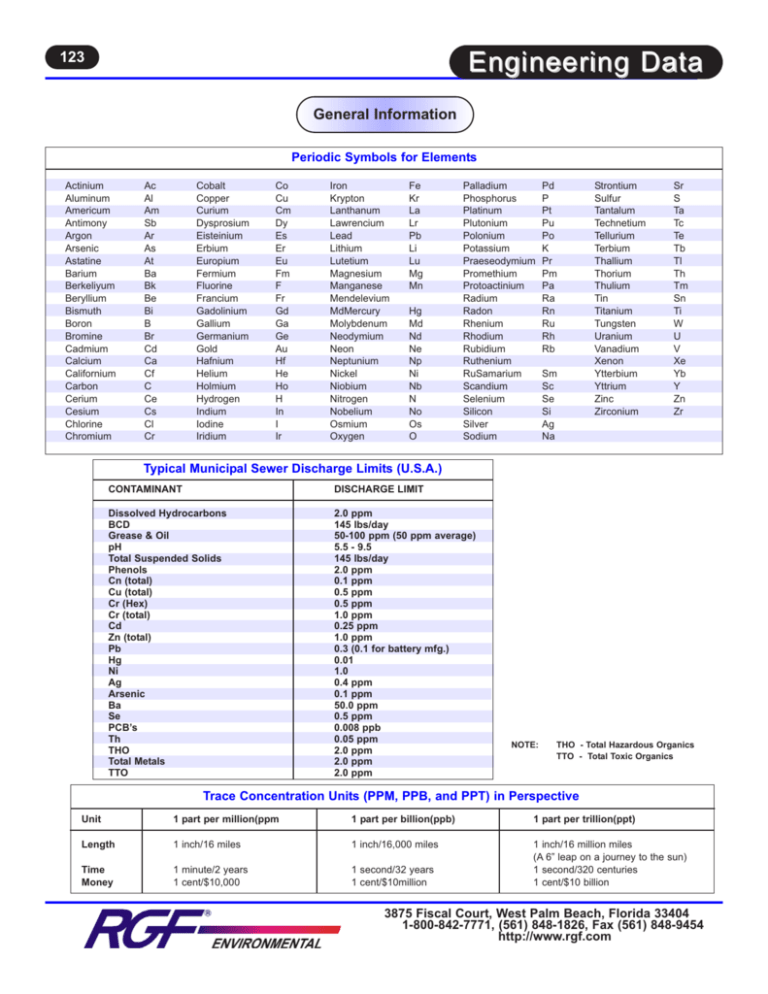
123 Engineering Data General Information Periodic Symbols for Elements Actinium Aluminum Americum Antimony Argon Arsenic Astatine Barium Berkeliyum Beryllium Bismuth Boron Bromine Cadmium Calcium Californium Carbon Cerium Cesium Chlorine Chromium Ac Al Am Sb Ar As At Ba Bk Be Bi B Br Cd Ca Cf C Ce Cs Cl Cr Cobalt Copper Curium Dysprosium Eisteinium Erbium Europium Fermium Fluorine Francium Gadolinium Gallium Germanium Gold Hafnium Helium Holmium Hydrogen Indium Iodine Iridium Co Cu Cm Dy Es Er Eu Fm F Fr Gd Ga Ge Au Hf He Ho H In I Ir Iron Krypton Lanthanum Lawrencium Lead Lithium Lutetium Magnesium Manganese Mendelevium MdMercury Molybdenum Neodymium Neon Neptunium Nickel Niobium Nitrogen Nobelium Osmium Oxygen Fe Kr La Lr Pb Li Lu Mg Mn Hg Md Nd Ne Np Ni Nb N No Os O Palladium Phosphorus Platinum Plutonium Polonium Potassium Praeseodymium Promethium Protoactinium Radium Radon Rhenium Rhodium Rubidium Ruthenium RuSamarium Scandium Selenium Silicon Silver Sodium Pd P Pt Pu Po K Pr Pm Pa Ra Rn Ru Rh Rb Sm Sc Se Si Ag Na Strontium Sulfur Tantalum Technetium Tellurium Terbium Thallium Thorium Thulium Tin Titanium Tungsten Uranium Vanadium Xenon Ytterbium Yttrium Zinc Zirconium Sr S Ta Tc Te Tb Tl Th Tm Sn Ti W U V Xe Yb Y Zn Zr Typical Municipal Sewer Discharge Limits (U.S.A.) CONTAMINANT DISCHARGE LIMIT Dissolved Hydrocarbons BCD Grease & Oil pH Total Suspended Solids Phenols Cn (total) Cu (total) Cr (Hex) Cr (total) Cd Zn (total) Pb Hg Ni Ag Arsenic Ba Se PCB’s Th THO Total Metals TTO 2.0 ppm 145 lbs/day 50-100 ppm (50 ppm average) 5.5 - 9.5 145 lbs/day 2.0 ppm 0.1 ppm 0.5 ppm 0.5 ppm 1.0 ppm 0.25 ppm 1.0 ppm 0.3 (0.1 for battery mfg.) 0.01 1.0 0.4 ppm 0.1 ppm 50.0 ppm 0.5 ppm 0.008 ppb 0.05 ppm 2.0 ppm 2.0 ppm 2.0 ppm NOTE: THO - Total Hazardous Organics TTO - Total Toxic Organics Trace Concentration Units (PPM, PPB, and PPT) in Perspective Unit 1 part per million(ppm 1 part per billion(ppb) 1 part per trillion(ppt) Length 1 inch/16 miles 1 inch/16,000 miles Time Money 1 minute/2 years 1 cent/$10,000 1 second/32 years 1 cent/$10million 1 inch/16 million miles (A 6” leap on a journey to the sun) 1 second/320 centuries 1 cent/$10 billion 3875 Fiscal Court, West Palm Beach, Florida 33404 1-800-842-7771, (561) 848-1826, Fax (561) 848-9454 http://www.rgf.com 124 Engineering Data Conversion Factors 1 mg/L (ppm) 17.118 mg/L 1 grain/gallon 1 million gallons/day 1 mg/L 1 gr/gallon (17.1 mg/L) 1 cubic inch 1 cubic centimeter 1 liter 1 quart 1 cubic foot 1 U.S. gallon 1 Imperial gallon 1 cubic foot water 1 1 1 1 1 1 inch foot centimeter yard square centimeter square inch Multiply Atmospheres Barrels of Oil Centimeters Centimeters Cubic Centimeters Cubic Centimeters Cubic Feet Cubic Feet Cubic Feet/min. Cubic Inches Cubic Inches Cubic Inches Cubic Meters Cubic Meters Feet Feet Feet of Water Feet of Water Gallons Gallons Gallons Gallons Gallons/min. Inches Inches of Hg. Inches of Hg. = = = = = = = = = = = = = = = = = = = = = = = = = = = = = = = = = = = 8,345 lbs/million gal 1 grain/gallon 142,857 lbs/million gal 694.4 gal/minute 2.6 grams/minute 0.0926 ounces/minute 0.347 pounds/hour 8.345 pounds/day 44.9 grams/minute 1.58 ounces/minute 5.925 pounds/minute 142.20 pounds/minute 16.39 cubic centimeters 0.061 cubic inch 61.02 cubic inches 0.0353 cubic feet 0.2642 U.S. gallon 0.9463 liter 28.32 liters 8.345 pounds 3.785 liters 231 cubic inches 0.1337 cubic foot 0.8327 Imperial gallon 4 quarts 1.2 U.S. gallons 62.425 pounds 7.4805 U.S. gallons 28.32 liters 2.54 centimeters 0.3048 meter 0.3937 inch 0.9144 meter 0.155 square inch 6.452 square centimeters By To Obtain 14.7 42.0 .03281 .3937 .06102 .0002642 7.4805 .1728 7.4805 .004329 16.387 .0005787 264.17 35.31 30.48006 .3048006 .4335 .8826 3,785.43 231 .83268 .13368 .13368 .0254 1.133 .491 psi gallons (U.S.) feet inches cubic inches gallons (liq.) gallons (liq.) cubic inches g.p.m. gallons cubic cm. cubic ft. gallons (liq.) cubic ft. centimeters meters psi Inches of Hg. cubic cm. cubic inches gallons (imp.) cubic feet cu. ft./min. meters feet of water psi 10,000 mg/L 1,000 mg/L 100 mg/L =1.0% = 0.1% = 0.01% 1 square meter 1 square foot 1 pound water = = = = = = = = = = = = = = = = = = = = = = = = = = = = 1 gram 1 kilogram 1 ounce 1 pound 1 short ton 1 metric ton 1 long ton 1 inch mercury 0.8826 inches of mercury 1 pound/square inch 0.4334 pounds/sq. inch Centigrade temperature Fahrenheit temperature 24 hours Multiply 10.76 square feet 0.0929 square meter 0.016 cubic foot 27.648 0.1198 U.S. gallons 0.4534 liter 15.43 grains 0.0353 ounce 2.205 pounds 28.35 grams 7,000 grains 0.4536 kilogram 453.6 grams 16 ounces 2,000 pounds 907.18 kilograms 2,205 pounds 2,240 pounds 1,133 feet of water 0.4912 lbs/sq. inch 0.0332 atmospheres 345.3 kilograms/sq. meter 1 foot of water 2,307 feet of water 1 foot of water (Fahrenheit-32) x 5/9 (Centigrade x 1.8) + 32 1,440 minutes By 2.2046 14.2233 1,422.33 .264178 3.2808 .11985 2.036 2.31 6.451 Kilograms Kilograms/sq. cm. Kilograms/sq. mm. Liters Meters Pounds Water PSI PSI Square Inches To Obtain pounds (avdp.) psi psi gallons feet gallons Inches of Hg. feet of water sq. cm Head 1 lb per sq. in 1 ft. of Water 1 Atmosphere = = = = 2.31ft. Head of Water 0.4333 lbs per sq. in. 14.7 lbs per sq. in. 33.95 ft. of water = = = = = = = = = = = = 1.0 .68 .74 .78 .82 .82 .88 .91 .91 .96 .90 1.0 Specific Gravity Water Gasoline Jet Fuel Kerosene Fuel Oil Diesel Fuel Sea 10 thru 250 Turbine Olive Castor Lard Navy No. 2 - .74 .85 .82 .95 .95 .93 3875 Fiscal Court, West Palm Beach, Florida 33404 1-800-842-7771, (561) 848-1826, Fax (561) 848-9454 http://www.rgf.com 125 Engineering Data Particle Sizes Comparative Particle Size Natural Settling Diameter of particle, mm Order of Size Time required to settle* 10 1 0.1 0.01 0.001 0.0001 0.00001 Gravel Course sand Fine sand Silt Bacteria Colloidal particles Colloidal particles 0.3 seconds 3 seconds 38 seconds 33 minutes 55 hours 230 days 6.3 years *Calculations based on spere with a specific gravity of 2.65 to settle 1 ft. Particle Sizes (Microns) 1 Micron = 1 Micrometer = 1 Millionth of a Meter 0.0001 0.001 0.01 DISSOLVED SOLIDS U.S. Mesh Inches Microns U.S. Mesh Inches Microns 3 3 1/2 4 5 6 7 8 10 12 14 16 18 20 25 30 35 6730 5660 4760 4000 3360 2830 2380 2000 1680 1410 1190 1000 841 707 595 500 40 45 50 60 70 80 100 120 140 170 200 230 270 325 400 420 354 297 250 210 177 149 125 105 88 74 63 53 44 37 0.1 .265 .223 .187 .157 .132 .111 .0937 .0787 .0661 .0555 .0469 .0394 .0331 .0280 .0232 .0197 1.0 COLLOIDAL SOLIDS .0165 .0138 .0117 .0098 .0083 .0070 .0059 .0049 .0041 .0035 .0029 .0024 .0021 .0017 .0015 10 100 SUSPENDED SOLIDS 1,000 SETTLEABLE SOLIDS MOLDS HAIR HOUSEHOLD DUST SAND BACTERIA VIRUS CRYPTOSPORIDIUM ASBESTOS POLLEN ANIMAL DANDER SUGARS SMOG PESTICIDES SPORE INSECTICIDE DUST SILTS DISSOLVED METALS AND SALTS PRECIPITATED METALS & PARTICULATE ELECTRON MICROSCOPE MICROSCOPE PARTICLE FILTRATION ULTRAFILTRATION REVERSE OSMOSIS VISIBLE BY HUMAN EYE CLARIFIER MOLECULAR SEPARATION SCREEN NANOFILTRATION 0.0001 Molecular Wt. 0.001 200 0.01 20,000 0.1 200,000 1.0 10 100 1,000 3875 Fiscal Court, West Palm Beach, Florida 33404 1-800-842-7771, (561) 848-1826, Fax (561) 848-9454 http://www.rgf.com 126 Engineering Data Value Engineering The objective of value engineering is to make a structured review of a project by a carefully selected team of engineers in order to identify potential savings in capital and/or operating costs. Studies conducted by the U.S. Naval Facilities Engineering Command, U.S. General Services Administration, and EPA have concluded that proper use of the value engineering concept can save an average of at least 10 percent of the cost of major projects. In value engineering, the objective is to improve the relationship of worth to cost through the study of functions. Worth is the amount that is willing to be paid for a function, which is a purpose for which an item is identified. With this premise, the value engineering work plan systematically uses nine phases for cost or value analysis. I. II. III. Materials Technologies Process IV. V. VI. Redundancy Quality Safety VII. VIII. IX. Product Application Moral Projected Decline of Fresh Water Fresh Water Available vs. Population Growth TOTAL WATER SUPPLY Contaminated Water FACT Fresh Water There is not one more drop of water on earth today than there was when cave men inhabited the Earth! Fresh Water Population Growth Water Usage 1850 1996 National Industrial Water & Sewer Costs State, City GA, Atlanta AZ, Phoenix TX, Houston OR, Portland CA, Los Angeles ME, Portland IN, Indianapolis NY, New York Unit Cubic Feet 140 100 401 100 100 200 100 200 Unit in Gallons 1047 647 3000 748 748 1496 748 1485 Cost of Water per 1.65 1.01 12.08 1.14 1.94 1.92 1.07 1.01 Cost of Sewer per Water/Sewer Total per Unit 1.7 4.42 11.94 2.06 2.03 6.68 0.85 1.52 National Average Cost per Gallon (Water/Sewer) 3.35 5.43 24.02 3.2 3.97 8.6 1.92 2.53 Cost per Gallon Water & Sewer $0.0032 0.0073 0.0080 0.0043 0.0053 0.0057 0.0030 0.0017 $0.005 3875 Fiscal Court, West Palm Beach, Florida 33404 1-800-842-7771, (561) 848-1826, Fax (561) 848-9454 http://www.rgf.com 127 Engineering Data Value Engineering Risk vs. Benefits of Wash Water Recycling Truck / Heavy Equipment Washing Liability Discharge to Ground Wash Water Recycling • • • • • • • • • • • Illegal Activity Resulting in fines Clean-up Cost Loss of Real Estate Value Risk of Civil Litigation for Ground Water Pollution Negative Publicity Saves Water Saves Cleaning Chemicals Protects Real Estate Value Alleviates Litigation Liability Promotes a Positive Image Improves Employee Morale Pollutants Typical Equipment Pressure Cleaning Facility Soil Sample Typical State Discharge Limits • • • • • • • • • • • • 57,500 ppm 270 ppm 2.2 ppm 0.067 ppm 1.54 ppm 5,250 ppb 55,200 ppb 654,000 ppb 260,000 ppb 1,150 ppm 14 ppb 49,200 ppm 5 ppm* - dissolved or emulsified 0.003 ppm 5 ppb 0.2 ppb 0.05 ppm 5 ppb 5 ppb 400 ppb 5 ppb 1 ppm 0.05 ppb 0.5 ppm Oil & Grease Lead Cadmium Mercury Chromium Benzene Toluene Total Xylenes Methylene Chloride Barium Silver Detergents 3875 Fiscal Court, West Palm Beach, Florida 33404 1-800-842-7771, (561) 848-1826, Fax (561) 848-9454 http://www.rgf.com 128 Engineering Data Value Engineering Truck / Heavy Equipment Washing Cost of Discharge vs. Recycling Cost of Washing Heavy Equipment & Discharge to Sewer Cost of Washing Heavy Equipment & Recycling 1. Total Water Used (per vehicle) 233 Gals . . . . . . . . . . . .$1.16 2. Cleaning Chemicals . . . . . . . . . . . . . . . . . .3.24 3. Total Water Used (per vehicle) . . . . . . . . .$0.23 (46 Gals. - 187 Recycled) 4. Cleaning Chemicals . . . . . . . . . . . . . . . . . .1.62 (50% Recycled) 5. Recycle Equipment Operating & Maint. . . .0.57 6. Depreciation . . . . . . . . . . . . . . . . . . . . . . . .0.59 TOTAL COST . . . . .$4.40 TOTAL COST . . . . .$3.01 SAVINGS = $1.39 per Vehicle Notes: 1. 2. 3. 4. 5. 6. Based on a national average water/sewer rate of .005 cents per gallon. 233 gals. based on a national average of 10 Truckwash Operations surveyed nationally. Based on a water loss rate of 20% due to evaporation and drag out. Based on national average cleaning chemical cost of $3.77 per gallon. $200 per month for maintenance and assuming 350 vehicles would be cleaned monthly = .574 each vehicle. Based on $25,000 Equipment, capital cost 10 years straight line depreciation, and 350 vehicles cleaned monthly. Global Market for Environmental Products 700 $ Dollars (Billions) 600 500 400 300 200 100 0 1996 2000 2010 2025 2050 3875 Fiscal Court, West Palm Beach, Florida 33404 1-800-842-7771, (561) 848-1826, Fax (561) 848-9454 http://www.rgf.com 129 Engineering Data Water Treatment Technology Guide TECHNOLOGY GUIDE SUMMARY EQUIPMENT USAGE EQUIPMENT USAGE Activated Carbon Color BOD/COD Oil & Grease Metals Incline Plate Coalescers Oil Removal Solids Settling Skimmers Oil & Flotable Removal Prefiltration - Bag Filters SS Removal Particles - Indexing Paper Metal Particles Sludge Solids Floc Reverse Osmosis UF Permeate Polishing Desalting Absorption - Carbon - Clay - Resin UF permeate Polishing Trace Oil & Grease COD/BOD Color Phenols Reduction Dechlorination Cracking (heat, acid, salts) Oil Separation Emulsion Breaking Clarifier SS Removal Settling Biological Systems (aerobic/anaerobic) BOD/COD Sugars UF Pretreatment Grey Water Black Water Microbiological Control - Sodium Hypochlorite - Quat - Hydrogen Peroxide Kills Bacteria, Virus, Algae Thermal Oxidation - Incinerators UF/RO Concentrate BTU Recovery Sludge Ozone BOD/COD Reduction Sanitizing Phenols Reduction Cartridge Filtration Particle Removal Suspended Solids Ultraviolet Light BOD/COD Reduction Sanitizing of Recovered Permeates for Reuse Incline Screen Filtration Heavy Particles Sludge Chemical Treatment - pH adjustment - Peroxide - Sodium Metabisulfite - Chlorine Addition BOD/COD reduction Chlorine Reduction Sanitizing Centrifugal Separator Free Oil Fine Oil Sludge Suspended Solids Air Strippers Volatile Organic Dissolved Air Flotation Suspended Solids Oil/Water Fats Protein Byproducts Evaporators UF Permeate RO Retentate Aqueous Cleaners Residue Multi-Media Filtration Suspended Solids Ultrafiltration Flocculation Metals Oils Proteins Fats Metals Solids Oils Recycling Cutting Molecular Separation Membrane Suspended solidsRecycling Wash Water Catalytic Oxidation (Advanced Oxidation) Oxidizes Organics BOD/COD Metals Algae, Bacteria, Viruses pH Adjustment Clarifying Cone Tanks Metal Precipitation Emulsion Control Flocculation Solubility Floc Settling Equalization pH Adjust Solids Settling 3875 Fiscal Court, West Palm Beach, Florida 33404 1-800-842-7771, (561) 848-1826, Fax (561) 848-9454 http://www.rgf.com 130 Engineering Data Ozone Basics UV Produced Ozone vs. CD Produced Ozone • UV OZONE is ozone produced by a specific wavelength of ultraviolet light (185 nanometers) reacting with an oxygen molecule to form O3 much the way our ozone layer is generated by the UV light from the sun. • CD OZONE is ozone produced from an electrical arc such as a welder arc. The electric spark also reacts with O2 to create O3 much like the ozone created after a lightning strike. Specification Color Odor Oxidential Potential Density Stability Disinfection Oxidizer Concentration Ozone Produced by UV Ozone Produced by CD Pale Blue Detectable at 0.01 ppm in air 2.07V in alkaline solution 150% of oxygen Unstable gas 1/2 life of minutes reverts back to oxygen Destroys micro organism, cysts, viruses, bacteria 3,125 times faster than chlorine The third strongest oxidizer known below fluorine and hydroxyl radical Ozone is 150% stronger than chlorine 0.1% by weight Pale Blue Detectable at 0.01 ppm in air 2.07V in alkaline solution 150% of oxygen Unstable gas 1/2 life of minute reverts back to oxygen Destroys micro organism, cysts, viruses, bacteria 3,125 times faster than chlorine The third strongest oxidizer known below fluorine and hydroxyl radical Ozone is 150% stronger than chlorine 0.5% to 10% by weight UV Produced Ozone CD Produced Ozone Advantages • • • • • • High concentration (% by weight) • Smaller compact units • High mass transfer efficiency in water Disadvantages • Low concentration (% by weight) • High operating cost on high-end application • Units are large in size Low cost No air preparation required Low maintenance Low operating cost on low ozone product High ppm levels for air treatment • Air preparation required • Low ozone product unit cost is high • The concentrations can be fatal A simple value engineering analysis of the above charts will clearly indicate the following: • UV OZONE is superior for low-end applications, especially for air/odor control treatment where high ppm is more important than high grams per hour, and where UV ozone dilution is not a problem. Also, a soft ozone is much safer in large volume air treatment. It is also superior for recirculating water applications under 10 gpm, or when UV light is used to enhance the ozone to create a hydroxyl radical as in the catalytic oxidation process. • CD OZONE is superior for high-end applications such as hazardous waste treatment or single pass high flow rates over 10 gpm requiring higher ozone doses. CD’s high concentration will maximize the oxidation potential per bubble, which the lower concentration of UV ozone cannot do. CD ozone is preferred when residual ozone in water is required. The high ozone concentration produced by CD is very inefficient for the high CFM levels required for air treatment due to the dilution factor. In other words, why make a high concentration and then dilute it. 3875 Fiscal Court, West Palm Beach, Florida 33404 1-800-842-7771, (561) 848-1826, Fax (561) 848-9454 http://www.rgf.com 131 Engineering Data Ozone Basics The Reciprocity Law of Doses Low intensity ozone or ultraviolet light radiation applied for a comparatively long period of time has the same lethal effect to microorganisms as high intensity ozone or ultraviolet radiation applied for a comparatively short time. 100% 75% sity ion n t e Int dia a h Hig UV R or ne o z O % Reduction 50% sity tion dia Ra n nte I w Lo V rU o ne zo 25% O 0% 1 2 3 4 5 6 Time 3875 Fiscal Court, West Palm Beach, Florida 33404 1-800-842-7771, (561) 848-1826, Fax (561) 848-9454 http://www.rgf.com 132 Engineering Data Ozone Air Treatment OZONE REQUIRED (gm/hr) FOR PRESCRIBED DOSAGE IN PPM/VOLUME 10.0 me Vo l m um by e Vo lu by by pp pp m 4 5p pm 6.0 me Vo lu pm b 10 p e lum o yV 3 Ozone Required, (gm/hr) y Vo lume 8.0 4.0 2 m pp b e m 2.0 1 pp olum by V me by Volu m p p 0.5 0.0 0 200 400 600 800 1000 Air Flow, (cfm @ 77O F & 1 atm) MASS TRANSFER RULES FOR OZONE IN AIR I. Temperature - The higher the temperature, the shorter the half-life of ozone or the lower the effectiveness of ozone. II. Concentration or Percentage by Weight - The higher the percentage of ozone in the carrier gas, the harder it is to dissipate the ozone for mass air treatment. 3875 Fiscal Court, West Palm Beach, Florida 33404 1-800-842-7771, (561) 848-1826, Fax (561) 848-9454 http://www.rgf.com 133 Engineering Data Ozone Air Treatment Typical Odor Recognition Thresholds Compound ppm by volume Compound ppm by volume Acetaldehyde Acetic acid Acetone Acrolein Acrylonitrile Allyl chloride Ammonia Aniline Benzene Benzyl chloride Benzyl sulfide Bromine Butyric acid Carbon disulfide Carbon tetrachloride Chloral Chlorine O-Cresol Dimethylacetamide Dimethylformamide Dimethylsulfide Diphenyl sulfide Ethanol Ethyl acrylate 0.2 1 100 0.2 20 0.5 50 1 5 0.05 0.002 0.05 0.001 0.2 20 0.05 0.3 0.001 50 100 0.001 0.005 10 0.0005 Ethyl mercaptan Formaldehyde Hydrochloric acid Hydogen sulfide gas Methanol Methylene chloride Methylene ethyl ketone Methyl isobutyl ketone Methyl mercaptan Methyl methacrylate Monochlorobenzene Nitroenzene Perchlorethylene Phenol Phosgene Phosphine Pyridine Styrene Sulfur dichloride Sulfur dioxide Toluene Trichloroethylene p-Xylene 0.001 1 10 0.0005 100 200 10 0.5 0.002 0.2 0.2 0.005 5 0.05 1 0.02 0.02 0.05 0.001 0.5 5 20 0.5 TYPICAL INDUSTRIAL ODOR CONTROL DOSAGES INDUSTRY ODOR APPLICATION DOSE • • • • Smoke Hydrogen Sulfide Fermentation Odors Cooking Odors (food) Direct Contact Exhaust Gas Contact Building Exhaust Kitchen Exhaust, Pan System Exit of a Cyclone Collector 40 ft. from top of Discharge Stack Recovery Furnace Exhaust 0.5 1.0 1.0 1.0 Smoke Fire Damage Sewer Lift Station Food Processing Cooking (Residential & Commercial) • Rubber Plant Processing Odors • Fishery Wet Scrubber Exhaust • Pulp Mill • Compost/Waste Management • Marine Hydrogen Sulfide/ Sulfur Dioxide Ammonia and Sulfur Compounds Bilge, Diesel Fumes • Rendering • Organic Organic and Chemical Ammonia and Sulfur Exhaust Gas Contact Chamber Direct Bilge Engine Room Contact Building Exhaust Building Exhaust ppm ppm ppm ppm RETENTION TIME to to to to 1.5 ppm 2.0 ppm 2.0 ppm 2 ppm N/A N/A 60 seconds N/A 2.0 ppm to 4.0 ppm N/A 5.0 ppm to 10.0 ppm N/A 60.0 ppm to 80.0 ppm N/A 2.0 ppm to 5.0 ppm 0.5 ppm to 1.5 ppm 45 second hold up time N/A 94.0 ppm 5.0 ppm 5 seconds 30 seconds Formula for Calculating Air Treatment Times Example for Model 400 / 900 Calculate Area to be treated: Length x Width x Height of room = Cubic Foot Cubic Ft. / cfm = Minutes needed to turn air in room over 1 time. 10’L x 12’W x 8’H room = 960 cubic feet 960 cu. ft. / 45 cfm = 21 minutes to turn room over 1 time. The amount of time necessary to sterilize an area with ozone depends upon the temperature, humidity level, and the amount of reactive substances (odors). 3875 Fiscal Court, West Palm Beach, Florida 33404 1-800-842-7771, (561) 848-1826, Fax (561) 848-9454 http://www.rgf.com 0.01 0.001 Microns 0.0001 1.0 104 Silts 0.1 1.0 Microscopic 10 CryptoSporidium 100 106 Pollen Hairs 1000 107 Sand 100 1000 Visible to Human Eye Foam Filters Ultra Filters HEPA Filters Fiber Particle Filters Spores Molds Bacteria 10 105 500,000mW Asbestos Household Dust 0.1 1000A 100,000mW Ozone Electrostatic Negative Ions Pesticides Smog Electron Microscope Gases Virus 0.01 Optical Analysis Filtration And Oxidation Technologies Air Pollutants 0.001 0.0001 Microns 100A 1000mW Odors 10A 1A Angstroms 100mW 1mW Molecular Weight Air Filtration Spectrum Engineering Data 134 Ozone Air Treatment 3875 Fiscal Court, West Palm Beach, Florida 33404 1-800-842-7771, (561) 848-1826, Fax (561) 848-9454 http://www.rgf.com 135 Engineering Data Ozone Water Treatment SOLUBILITY OF OZONE IN WATER UV PRODUCED / CD PRODUCED OZONE UV Produced O3 No Air Pretreatment Temperature CD Produced O3 Air Pretreatment (Dried) UV 0.1% by Weight CD 1.0% by Weight CD 2.0% by Weight 5O C/41O F 0.74 ppm 7.39 ppm 14.79 ppm 25O C/77O F 0.35 ppm 3.53 ppm 7.05 ppm 30O C/86O F 0.27 ppm 2.70 ppm 5.39 ppm MASS TRANSFER RULES FOR OZONE IN WATER I. Concentration or ozone percentage by weight. -The higher the percentage of ozone in the carrier gas, the more ozone is absorbed into the water. II. Pressure - The higher the water pressure, the more gas (ozone) will be held by the water. III. Bubble Size - The smaller the bubble size, the more the surface area increases, thereby increasing the ozone to water interface for a higher mass transfer efficiency. IV. Temperature - The higher the water temperature, the less gas it holds; the lower the water temperature, the more gas (ozone) it will absorb. TYPICAL WATER TREATMENT DOSAGES APPLICATION OZONE PPM Disinfection (Potable) MicroFlocculation THM’s Tanning/Liquid (Potable) Taste & Odor Cooling Towers Wastewater Bottled Water Swimming Pools Hot Tubs/Spas Sea/Aquatic Life Fresh Water/Aquatic Life 1.5 - 3.0 1.0 - 3.0 1.5 - 3.0 .5 - 5.0 1.0 -5.0 .2 - .6 5.0 - 15.0 1.8 - 2.0 0.1 -0.5 0.1 - .75 0.5 0.5 Designing an Ozone Water Treatment System Example: CONTACT TIME 5 5 5 4 5 5 15 5 - 10 10 10 20 10 10 30 10 4 4 minutes minutes minutes minutes minutes minutes minutes minutes minutes minutes Varies Varies Ozone Calculations Example: Iron and Manganese contamination of well water. 1 ppm Iron (Fe) requires 0.43 ppm O3 1 ppm Manganese (Mn) requires 0.88 ppm O3 1. (Fe) x .43 (O3) .3 (Mn) x .88 (O3) Ozone Required Safety Factor x 1 Ozone Dose Problem: Iron and Manganese water contamination • Water flow 10 gpm • Iron (Fe) contamination 1.0 ppm • Manganese (Mn) contamination 0.3 ppm = .43 ppm = .27 ppm .70 ppm = .70 = 1.40 ppm 1.40 ppm (dose) x 10 gpm x 0.227 = 3.18 gr/hr Note: 0.277 = conversion factor 3875 Fiscal Court, West Palm Beach, Florida 33404 1-800-842-7771, (561) 848-1826, Fax (561) 848-9454 http://www.rgf.com 136 Engineering Data Advanced Oxidation Water Treatment COMMON OXIDANTS Oxidants Effectiveness Relative to Chlorine Fluorine Hydroxyl Radical* Ozone* Hydrogen Peroxide* Permanganate Chlorine Bromine Iodine Oxygen* ORP Redox Potential 2.23 2.06 1.78 1.31 1.24 1.0 .80 .39 .29 2.85 volts 2.80 volts 2.07 volts 1.52 volts 1.36 volts 0.40 volts * Elements that participate in the Catalytic Oxidation Process Advanced Oxidation Water Treatment Oxidation is a chemical reaction in which an element or ion is increased in positive valence, losing electrons to an oxidizing agent. To oxidize is to change a substance by chemical reaction by combining it with oxygen, such as fire or rust. By utilizing Redundant Oxidizers, the speed and efficiency of the oxidation process is greatly increased, in some cases over 40 times. Oxidants considerably stronger than chlorine include Hydroxyl Radical (OH0), Ozone (O3) and Hydrogen Peroxide (H202). These oxidizers are preferred for potable water treatment or water recycling as they leave no dissolved byproducts as chlorine does, OH0, O3 and H2O2 consist of hydrogen and oxygen. Oxidizing reactions of interest in pollutant removal is OH0 or Hydroxyl Radical, a byproduct of the Catalytic Oxidation process. OH0 is very unstable thereby making it very aggressive or a free radical. The Hydroxyl or free radical occurs when ozone or hydrogen peroxide reacts with UV radiation and protolysis occurs. Although the Hydroxyl Radicals are short lived, they have a higher oxidation potential than ozone, chlorine or hydrogen peroxide, and their unstable nature increases their reaction speed. A strong benefit of Catalytic Oxidation is the end products of CO2 and H2O, or Carbon Dioxide and Water. Catalytic Oxidation occurs with: O3 + UV = OH0 or O3 + H2O2 + UV = OH0 • For potable water treatment UV & O3 are used in our UV/O3 Catalytic Systems. • The addition of H2O2 is added to O3 & UV in our CO3P Catalytic Oxidation Systems and is highly recommended for wastewater treatment. OXIDATION HALF-LIFE OF VARIOUS ORGANIC COMPOUNDS Compound Chlorine Permanganate Ozone Acetophenone Benzaldehyde Camphor Ethylbenzene 1, 2, 3 - Trimethylbenzene 26 days >3.2 days >3.2 days N/A N/A 43 days 36 minutes >5.8 days N/A N/A 25 minutes 28 minutes >12 minutes 2.8 minutes 1.9 minutes 3875 Fiscal Court, West Palm Beach, Florida 33404 1-800-842-7771, (561) 848-1826, Fax (561) 848-9454 http://www.rgf.com 137 Engineering Data UV Light Water Treatment UV Dose for 99.9% Reduction Bacteria & Viruses Dysentery E-Coli Infectious Jaundice Typhoid Fever Hepatitis Virus Influenza Virus Tuberculosis Bacillus Anthracis Polio Virus 0 5,000 10,000 15,000 20,000 25,000 UV Dosage (microwatt-secs/cm2) Microwatts - secs/cm2 UV ENERGY IN MICROWATT - SECONDS PER SQUARE CENTIMETER Note: U.S. Department of Health recommends 16,000 microwatt - sec/CM2 for potable water. Catalytic Oxidation Formula UV Dosage Formula UV/ID UV ID IT Epv mW/cm2 RT mW-sec/cm2 = = = = = = = = IT x Epv = (MW/CM2) x RT = MW-Secs/CM2 Ultraviolet Light Intensity Dosage Intensity Transmission (Lamp Energy) Exposure per Volume (Water Volume Treated) Total UV Energy Output (Full Lamp length) Retention Time (Volume of Water Passing Lamp) Micro Watt - Seconds per Square Centimeter O3 + UV + H2O2 HR + Wastewater Treatment O3 + UV HR + Potable Water Treatment Where, O3 = Ozone UV = Ultraviolet Light H2O2 = Hydrogen Peroxide HR = Hydroxyl Radical Note: UV is an effective method of killing bacteria in water as is ozone. Both methods are recognized as effective by the U.S. Department of Health. Combined, they create a hydroxyl radical or an extremely effective oxidation method. The RGF Catalytic Oxidation Process for potable water treatment utilizes O3 + UV = HR for three strong oxidizers. The RGF CO3P Systems for wastewater treatment utilize O3 + UV + H2O2 = HR for four strong oxidizers. 3875 Fiscal Court, West Palm Beach, Florida 33404 1-800-842-7771, (561) 848-1826, Fax (561) 848-9454 http://www.rgf.com