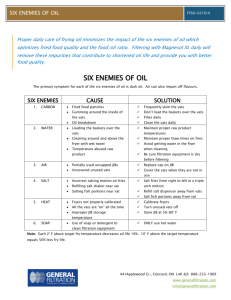
National Medical Policy
advertisement

National Medical Policy Subject: Video-Assisted Thoracoscopic Surgery (VATS) Policy Number: NMP430 Effective Date*: August 2008 Updated: September 2015 This National Medical Policy is subject to the terms in the IMPORTANT NOTICE at the end of this document For Medicaid Plans: Please refer to the appropriate Medicaid Manuals for, coverage guidelines prior to applying Health Net Medical Policies The Centers for Medicare & Medicaid Services (CMS) For Medicare Advantage members please refer to the following for coverage guidelines first: Use X Source National Coverage Determination (NCD) Reference/Website Link Lung Volume Reduction Surgery (Reduction Pneumoplasty) (240.1): http://www.cms.gov/medicare-coveragedatabase/search/advanced-search.aspx X National Coverage Manual Citation Local Coverage Determination (LCD)* Article (Local)* Other None CMS Manual System Department of Health & Human Services (DHHS). Pub. 100-03 Medicare National Coverage Determinations. Transmittal 3: https://www.cms.gov/Regulations-andGuidance/Guidance/Transmittals/downloads/R3NCD.p df Use Health Net Policy Instructions Medicare NCDs and National Coverage Manuals apply to ALL Medicare members in ALL regions. Medicare LCDs and Articles apply to members in specific regions. To access your specific region, select the link provided under “Reference/Website” and follow the search instructions. Enter the topic and your specific state to find the coverage determinations for your region. *Note: Health Net must follow local coverage determinations (LCDs) of Medicare Administration Contractors (MACs) located outside their service area when those MACs have exclusive coverage of an item or service. (CMS Manual Chapter 4 Section 90.2) If more than one source is checked, you need to access all sources as, on occasion, an LCD or article contains additional coverage information than contained in the NCD or National Coverage Manual. Video Assisted Thoracoscopic Surgery (VATS) Sep 15 1 If there is no NCD, National Coverage Manual or region specific LCD/Article, follow the Health Net Hierarchy of Medical Resources for guidance. Current Policy Statement Please refer to the Health Net policy, Lung Volume Reduction Surgery (LVRS) and the Health Net policy on Hyperhidrosis Treatments Health Net, Inc. considers video-assisted thoracoscopic surgery (VATS) medically necessary for diagnostic and therapeutic pleural, lung, and mediastinal surgery. Many procedures historically performed as an open thoracotomy are now performed as VATS. Some examples of procedures that can be performed by a VATS approach include, but not limited to the following: Biopsy (e.g. , mediastinal nodes, wedge resection lung, lesions of the pleura, diaphragm, lung, pericardium, esophagus, spine) Resection (e.g., blebectomy, pneumonectomy, segmentectomy, esophagectomy, thymectomy) Drainage (e.g., pleural, mediastinal abscess, pericardial window) Pulmonary decortication Pleurodesis Pericardial procedures (e.g., pericardial window) Thoracic sympathectomy As an alternative to thoracotomy in individuals who meet the criteria specified in the Health Net policy, Lung Volume Reduction Surgery (LVRS). Contraindications Contraindications to VATS are related to pulmonary disease (pulmonary insufficiency) or anatomic limitations, such as fusion of the visceral and parietal pleura, and thick fibrotic adhesions that obscure exposure and risk injury to vessels and structures. Individuals who cannot tolerate selective lung ventilation due to pulmonary insufficiency are not ideal candidates for VATS. Other contraindications include: Inability to perform a complete (R0) resection with suitable residual cardiopulmonary reserve Lymph node metastasis beyond regional lymph nodes Widely metastatic disease Definitions VATS NSCLC SIGN LVRS BLVRS PFT PH PLH CH Video-Assisted Thoracoscopic Surgery Non-small cell lung cancer Scottish Intercollegiate Guidelines Network Lung Volume Reduction Surgery Bilateral Lung Volume Reduction Surgery Pulmonary function test Palmar hyperhidrosis Plantar hyperhidrosis Compensatory hyperhidrosis Codes Related To This Policy NOTE: The codes listed in this policy are for reference purposes only. Listing of a code in this policy does not imply that the service described by this code is a covered or noncovered health service. Coverage is determined by the benefit documents and medical necessity criteria. This list of codes may not be all inclusive. Video Assisted Thoracoscopic Surgery (VATS) Sep 15 2 On October 1, 2015, the ICD-9 code sets used to report medical diagnoses and inpatient procedures will be replaced by ICD-10 code sets. Health Net National Medical Policies will now include the preliminary ICD-10 codes in preparation for this transition. Please note that these may not be the final versions of the codes and that will not be accepted for billing or payment purposes until the October 1, 2015 implementation date. ICD-9 Codes (List is not all inclusive) 162.9 780.8 492.0 492.8 511.9 Primary neoplasm of Lung Hyperhidrosis Emphysematous bleb Other emphysema Unspecified pleural effusion ICD-10 Codes (List is not all inclusive) C34.90 J43-J43.9 J44-j44.9 R61 R91.0 Malignant neoplasm of unspecified part of unspecified bronchus or Lung Emphysema Other chronic obstructive pulmonary disease Generalized hyperhidrosis Solitary pulmonary nodule CPT Codes 32601-32665 32666 32667 32668 32669 32670 32671 32672 32673 32674 Thoracoscopy Thoracoscopy, surgical with therapeutic wedge resection (e.g. mass, nodule) initial, unilateral Thoracoscopy, surgical with therapeutic wedge resection (e.g. mass, nodule), each additional resection, ipsilateral Thoracoscopy, surgical with diagnostic wedge followed by anatomic lung resection Thoracoscopy, surgical with removal of a single lung segment Thoracoscopy, with removal of two lobes (lobectomy) Thoracoscopy, with removal of lung (pneumonectomy) Thoracoscopy, with resection-plication for emphysematous or nonbullous) for lung volume reduction (LVRS), unilateral includes any pleural procedure, when performed Thoracoscopy, with resection of thymus, unilateral or bilateral Thoracoscopy, with mediastinal and regional lymphadenectomy HCPCS Codes N/A Scientific Rationale – Update September 2015 NCCN guidelines on NSCLC (Version 7.2015) state that video-assisted thoracic surgery (VATS), also known as thorascopic lobectomy, is currently being investigated in all aspects of lung cancer. They note that published studies suggest that thorascopic lobectomy has several advantages over the standard thoracotomy that includes less pain, shorter length of hospitalization, lower postoperative morbidity and mortality, minimal risk of intraoperative bleeding, or minimal locoregional recurrence. They also note that thorascopic lobectomy is associated with less morbidity, fewer complications, and more rapid return to function than lobectomy by thoracotomy. Per the NCCN guidelines, “In patients with stage I NSCLC who had thorascopic lobectomy with lymph node dissection, the 5-year survival rate, longVideo Assisted Thoracoscopic Surgery (VATS) Sep 15 3 term survival and local recurrence were comparable to those achieved by routine open lung resection. Thorascopic lobectomy has also been shown to improve discharge independence in older populations and in patients at high risk. Data show that thorascopic lobectomy improves the ability of patients to complete postoperative chemotherapy regimens. Based on its favorable effects on postoperative recovery and morbidity, thorascopic lobectomy (including robotic-assisted approaches) is recommended in the NCCN algorithm as an acceptable approach for patients who are surgically resectable (and have no anatomic or surgical contraindications) as long as standard principles of thoracic surgery are not compromised.” Wu et al (2015) reviewed and compared the perioperative outcomes of VATS with open thoracotomy for chest trauma patients. The authors conducted a systematic review and meta-analysis of randomized control trials and cohort studies comparing the perioperative outcomes of VATS with open thoracotomy for chest trauma patients. Clinical endpoints included postoperative complications, perioperative mortality rate, chest tube drainage volume, duration of tube drainage, duration of hospitalization, operation time, and amount of bleeding and transfusion volume in operation. A subgroup analysis was performed to explore the potential source of heterogeneity. Twenty-six studies were included. Pooled analyses showed significant reductions in the incidence of postoperative complications (risk ratio [RR] [95% confidence interval (CI)], 0.47 [0.35, 0.64]), chest tube drainage volume (mean difference [MD] [95% CI], -146.88 ml [-196.04, -97.72]), duration of tube drainage (MD, -1.71 days; 95% CI -2.16 to -1.26), duration of hospitalization (MD, -4.67 days; 95% CI -5.19 to-4.14), operation time (MD, -41.18 min; 95% CI -52.85 to 29.51), and amount of bleeding (MD, -119.10 ml; 95% CI -147.28 to -90.92) and transfusion volume (MD, -379.51 ml; 95% CI -521.24 to-237.77) in chest trauma patients treated with VATS compared with open thoracotomy. The perioperative mortality rate was not significantly different between patients received VATS and open thoracotomy (RR, 0.52; 95% CI 0.22-1.21). The authors concluded compared to open thoracotomy, VATS is an effective and even better treatment for improving perioperative outcomes of hemodynamically stable patients with chest trauma and reduce the complications. However, caution should also be exercised in certain clinical scenarios. Tane et al (2015) examined the advantages of thoracoscopy over thoracotomy in terms of perioperative outcomes and toleration of adjuvant chemotherapy. Between April 2010 and March 2013, 657 patients with NSCLC who underwent lobectomy were classified into thoracoscopy (308 patients) and thoracotomy (349 patients) groups and compared. The thoracoscopy group had less blood loss compared to the thoracotomy group (p<0.001). When limiting the analysis to pathological stage I patients, the results were similar (p<0.001). In addition, the difference in blood loss between the 2 groups was greater in patients with severe pleural adhesions. The postoperative morbidity of the thoracoscopy group was significantly less than that of the thoracotomy group (13.3% vs. 21.2%, p<0.001), and this result was similar when analyzing the pathological stage I patients (12.6% vs. 20.6%, p=0.001). A higher percentage of the thoracoscopy group received both the full planned course and dose of adjuvant chemotherapy compared to the thoracotomy group (84.2% vs. 65.8%, p=0.032). The authors concluded the results indicate that totally thoracoscopic lobectomy is the more beneficial surgical approach with regard to the incidence of postoperative complications and toleration of adjuvant chemotherapy. Laursen et al (2015) compared the 30-day morbidity and mortality for lung cancer patients operated by VATS lobectomy or lobectomy by open thoracotomy (OT). Data were obtained from prospective national and regional databases, including patients who underwent lobectomy for lung cancer in the eastern part of Denmark from 1 January 2005 to 31 December 2011. All patients operated before 2009 were reVideo Assisted Thoracoscopic Surgery (VATS) Sep 15 4 staged according to the latest International Association for the Study of Lung Cancer lung cancer classification. Patient characteristics, comorbidities, pathology and operative data were assessed using an independent samples t-test, Pearson's χ2, Fisher's exact test and Mann-Whitney test. Morbidity was assessed using multinomial logistic regression adjusted for gender, age, cancer stage, forced expiratory volume in 1 s (FEV1), year of surgery and Charlson comorbidity score. In total, 1379 patients underwent lobectomy, 785 patients via VATS and 594 patients via thoracotomy. The two groups were similar in gender and FEV1. The patients operated by VATS were older (P < 0.001), and had a lower Charlson comorbidity score (P = 0.034), higher frequency of adenocarcinomas (P < 0.001) and lower cancer stage (P < 0.001). Among the VATS patients, 285 (36.3%) and among the thoracotomy patients, 288 (48.5%) had minor complications (P < 0.001); and 157 (20.0%) VATS patients and 212 (35.7%) thoracotomy patients had major complications (P < 0.001). The 30-day mortality rate was 1% in the VATS group and 1.5% in the thoracotomy group (P = 0.47). Multinomial logistic regression analysis showed that the prevalence of both minor [odds ratio (OR) = 1.51; 95% confidence interval (Cl) = 1.18-1.96] and major complications (OR = 1.91, 95% Cl = 1.44-2.53) was significantly higher for patients who underwent lobectomy via thoracotomy compared with VATS. The authors concluded patients undergoing lobectomy via VATS were less likely to have at least one minor complication within the first 30 postoperative days and less likely to have at least one major complication, compared with patients operated by thoracotomy. These findings remained after adjusting for gender, age, FEV1, cancer stage, year of surgery and Charlson comorbidity score. Scientific Rationale – Update September 2014 Video assisted thoracoscopic surgery (VATS) is a set of minimally invasive thoracic surgical procedures principally employed in the management of pulmonary, mediastinal, and pleural pathology. Its main benefit has been the avoidance of a thoracotomy incision, which allows a shorter operative time, less postoperative morbidity, and earlier return to normal activity than with thoracotomy. Many procedures historically performed as an open thoracotomy are now performed as VATS although VATS is a technically demanding procedure. The advantage of VATS, when compared with open thoracotomy, include reduced hospital length of stay, less blood loss, less pain, improvement in pulmonary function when compared with open thoracotomy, early patient mobilization with early recovery and rapid return to work and daily activities, and less inflammatory reaction, as measured by cytokine response in patients undergoing VATS lobectomy compared with open thoracotomy. VATS is the procedure of choice for the diagnosis and management of diseases of the pleura, nondiagnosed peripheral pulmonary nodules, and interstitial lung disease. Today it is a well-accepted and established procedure and has become the first choice technique for lung biopsies, pleurectomies, sympathectomies, and other various pulmonary disorders. NCCN guidelines on NSCLC (Version 4.2014) state that VATS, also known as thorascopic lobectomy, is recommended as an acceptable approach for patients who are surgically resectable (and have no anatomic or surgical contraindications) as long as standards principles of thoracic surgery are not compromised. They note the surgical procedure used depends on the extent of the disease and on cardiopulmonary reserve of the patient. Ren et al (2014) compared the short-term effect of anatomic VATS segmentectomy and VATS lobectomy. From January 2011 to December 2012, 21 patients underwent VATS segmentectomy and 61 underwent VATS lobectomy. Intraoperative blood loss, operating time, postoperative drainage time, length of hospital stay, postoperative complications, local recurrence, and survival were compared between the two Video Assisted Thoracoscopic Surgery (VATS) Sep 15 5 groups. The intraoperative blood loss and average hospital stay were less in the segmentectomy group than in the lobectomy group (P<0.05). There was no significant difference in the operating time, number of lymph nodes dissected, postoperative drainage time, or 1-year survival between the two groups (P>0.05). Only one patient died because of heart disease. The two groups had a similar incidence of postoperative complications (P>0.05). There was one (4.8%) local recurrence after segmentectomy and two (3.3%) after lobectomy (P>0.05). The authors concluded VATS segmentectomy could be performed safely and is a method with favorable 1-year survival. It may be the ideal surgical procedure for patients with solitary pulmonary nodules in early stage lung cancer, especially for those with limited cardiopulmonary reserve or significant comorbidities. Rizk et al (2014) performed a prospective cohort study to compare quality of life and pain scores during the first 12 months after VATS or open anatomic resection. Patients were prospectively enrolled from May 2009 to April 2012. Patients with clinical stage I lung cancer who were scheduled to undergo anatomic lung resection were eligible. The Brief Pain Index and Medical Outcomes Study 36-Item Short Form Health Survey were conducted perioperatively and at four assessments during the first 12 months after the operation. Intent-to-treat analyses using mixed-effects models were used to longitudinally assess the effect of treatment on quality of life components (physical component summary and mental component summary) and pain. In total, 74 patients underwent thoracotomy, and 132 underwent VATS (including 19 patients who were converted to thoracotomy); 40 and 80 patients, respectively, completed the 12-month surveys. Baseline characteristics were similar between the two groups. Physical component summary and Brief Pain Index scores were similar between the two groups throughout the 12 months of follow-up. The mental component summary score, however, was consistently worse in the VATS group. Investigators concluded patient-reported physical component summary and pain scores after VATS and thoracotomy were similar during the first 12 months after surgical resection. Zhang et al (2014) performed a systemic review to investigate whether VATS could achieve equivalent lymph node (LN) evaluation efficacy to thoracotomy. A comprehensive search of PubMed, EMBASE, and Cochrane was performed to identify studies comparing VATS and thoracotomy in LNs and node stations. Mean difference was calculated by Review Manager 5.0 software and Stata 12. Twenty-four studies met the inclusion criteria of LN evaluation. 2,015 patients were involved in VATS group in contrast to 3,250 patients in thoracotomy group. The same number of total nodes stations (mean difference, 0.09; 95 % CI -0.25 to 0.42; P = 0.61) and mediastinal node stations (mean difference, -0.11; 95 % CI -0.24 to 0.01; P = 0.08) could be assessed by thoracotomy and VATS. The same number of N1 LNs (mean difference, -0.33; 95 % CI -0.70 to 0.05; P = 0.09) could be assessed by both groups. While more total (mean difference, -1.41; 95 % CI -1.99 to -0.83; P < 0.00001) and mediastinal LNs (mean difference, -1.03; 95 % CI -1.81 to -0.24; P = 0.01) could be harvested by thoracotomy. The authors concluded the outcomes show that the same number of total and mediastinal LN stations could be harvested by VATS and OT. The same number of N1 LNs could be harvested by VATS and OT, while less total and mediastinal LNs could be harvested by VATS. Yang et al (2014) sought to identify the risk factors for major adverse events of VATS lobectomy for primary lung cancer. 1806 Patients (1032 males, 57.1%) planned to undergo VATS lobectomy for stage IA-IIIA lung cancer from July 2007 to June 2012. The Thoracic Morbidity and Mortality Classification TM&M system was used to evaluate the presence and severity of complications. Postoperative complications were observed during a 30-day follow up. Univariate and multivariate analysis were used to analyze the independent risk factors for major adverse events. Video Assisted Thoracoscopic Surgery (VATS) Sep 15 6 Successful rate of VATS lobectomy was 97.6% (1763/1806). Major complications occurred in 129 patients (7.3%), with a mortality of 0.3% (5/1763). Pulmonary complications contribute up to 90.7% of the major complications and 80% of mortality. Logistic regression indicated that comorbidities, elder age ≥70y, operative time ≥240min and hybrid VATS were predictors for major adverse events (P<0.05). Hybrid and converted VATS lobectomy result in higher major adverse events compared with complete VATS, 15.1%, 20.9% and 7.4% respectively (P=0.013). Investigators concluded the overall complication rate and mortality of VATS lobectomy are low, while major complications sometimes occur. Pulmonary complications are the most common major complications and cause of mortality. Age ≥70y, comorbidities, operative time ≥240min and Hybrid VATS are predictors of major adverse events. Higuchi et al (2014) retrospectively compared long-term outcomes after VATS lobectomy versus lobectomy via open thoracotomy for clinical stage IA non-small cell lung cancer (NSCLC). From July 2002 to June 2012, 160 patients were diagnosed with clinical stage IA NSCLC and underwent lobectomy. Of these, 114 underwent VATS lobectomy and 46 underwent lobectomy via open thoracotomy. The 5-year disease-free survival (DFS) rate was 88.0% in the VATS group and 77.1% in the thoracotomy group for clinical stage IA NSCLC and 91.5% in the VATS group and 93.8% in the thoracotomy group for pathological stage IA NSCLC. The 5-year overall survival (OS) rate was 94.1% in the VATS group and 81.8% in the thoracotomy group for clinical stage IA NSCLC and 94.8% in the VATS group and 96.2% in the thoracotomy group for pathological stage IA NSCLC. The rate of accurate preoperative staging was 71.9% in the VATS group and 56.5% in the thoracotomy group. Inconsistencies between the clinical and pathological stages were mainly related to tumor size, nodal status, and pleural invasion. Local recurrence occurred for one lesion in the VATS group and six lesions (five patients) in the thoracotomy group. The reviewers concluded the DFS and OS were not inferior after VATS compared with thoracotomy. Local control was significantly better after VATS than after thoracotomy. Preoperative staging lacked sufficient accuracy. Gu et al (2014) evaluated the feasibility and safety of (VATS, and to compare the surgical results of VATS with the standard median sternotomy (MS) approach. Between April 2010 and April 2012, the data of 245 patients who underwent thymectomy for thymic tumors were prospectively collected. Among them, 93 patients with clinical stage I-II disease were retrospectively reviewed. Resection was planned for VATS in 49 cases, and for MS in 44 cases. During operation, there were 3 conversions to open surgery because of local invasion (conversion to thoracotomy in 1 patient, and sternotomy in 2). No transfusion was required in any patient. There was no significant difference in duration or amount of postoperative chest tube drainage between the 2 groups (P>0.05). Operative time, blood loss during operation, average length of the intensive care unit stay, and length of hospital stay were significantly less in the VATS group than the MS group (P<0.05). There were no major perioperative complications or mortality. No recurrence was detected during a median follow-up of 27 months (range, 12 to 36 mo). Reviewers concluded VATS thymectomy for early-stage thymic tumors is safe and feasible. In comparison with standard MS, the VATS approach was associated with a shorter intensive care unit stay and hospital stay. Prospective randomized multi-institutional trials with longterm follow-up are needed to compare the oncological outcomes. Manoly et al (2014) compared short- and mid-term outcomes for patients undergoing VATs or trans-sternal resection for thymoma in a feasibility study, highlights weaknesses in current research and makes recommendations for longterm technological evaluations in this field. Consecutive thymoma cases between 2004 and 2010 were identified. Patients were divided into two groups according to Video Assisted Thoracoscopic Surgery (VATS) Sep 15 7 surgical approach (Group I trans-sternal; Group II VATS) and comparisons were made between groups. The primary outcome was overall survival. Secondary outcomes included operative morbidity and mortality, hospital stay, recurrence rate and disease-free survival. Thirty-nine patients were included (Group I: n = 22 vs Group II: n = 17). There were no differences between groups at baseline for all measured covariates. No deaths occurred within 30 days of surgery. More patients in Group I developed complications (Group I: n = 10 vs Group II: n = 3; P = 0.093), while hospital stay was shorter in Group II (Group I: 6.4 ± 4.6 days vs Group II: 4.4 ± 1.8 days; P = 0.030). Five-year overall survival (Group I: 93.8 ± 6.1% vs Group II: 83.3 ± 11.2%; P = 0.425), 5-year disease-free survival (Group I: 71.0 ± 15.3% vs Group II: 83.3 ± 11.2%; P = 0.827) and recurrence rates at final follow-up (Group I: n = 2 vs Group II: n = 1; P = 0.363) were similar between the groups. Investigators concluded VATS thymectomy for thymoma is feasible, safe and has comparable mid-term oncological outcomes to trans-sternal thymectomy. Future research is required to evaluate long-term oncological outcomes of VATS thymectomy for thymoma in national registries and randomized, controlled trials. Boffa et al (2014) evaluated complications following anatomic resection of clinical stage I lung cancer by VATS and thoracotomy to clarify the effect of the minimally invasive approach. The Society of Thoracic Surgeons database was queried for lobectomies and segmentectomies performed between 2001 and 2010 for clinical stage I primary cancer. A total of 11,531 (7137 open and 4394 VATS) patients with clinical stage I primary lung cancers underwent resection. Propensity scoring was used to match cases into 2745 well-balanced pairs. Overall complications were significantly more likely in the thoracotomy group (36%) than in the VATS cohort (30%; P < .001). Patients undergoing thoracotomy experienced significantly more pulmonary complications (21% vs 18%), atrial arrhythmias (13% vs 10%), and were more likely to undergo transfusion (6% vs 4%). Operative mortality was similar (thoracotomy 1.8%, VATS 1.3%; P = .13). The authors concluded anatomic resection of early stage lung cancer is performed with a low mortality rate, according to data from the Society of Thoracic Surgeons database. Perioperative complications are significantly less likely to occur when patients with stage I lung cancers undergo resection using the VATS approach. Further study is warranted to determine longterm effects of these differences in perioperative outcomes Lin et al (2013) evaluated the risk and efficacy of wedge resection under VATS on elderly high-risk patients with severe cardiopulmonary and other system dysfunctions who are unable to tolerate pulmonary lobectomy with stage I peripheral non-small-cell lung cancer (PNSCLC). Elderly patients (≥70 years) with suspected PNSCLC were divided into high-risk group and conventional risk group. The high-risk patients confirmed in stage I by the examination of positron emission tomography computed tomography (PET-CT) and the postoperative patients in stage I PNSCLC with negative incisal margin were treated with VATS wedge resection. The conventional risk patients were treated with VATS radical resection and systematic lymphadenectomy. The clinical and pathological data were recorded. The total survival, tumor-free survival, recurrence time and style of patients were followed up. The operative time and blood loss of the VATS wedge resection group (69.4 ± 15.5 min, 52.1 ± 11.2 ml) were significantly less than those of the VATS radical resection group (128 ± 35.5 min, 217.9 ± 87.1 ml). Neither groups had postoperative death. The overall and tumor-free survival rate of the VATS wedge resection group within three years were 66.7% and 60.0%, and those of the VATS radical resection group were 93.8% and 94.1%, without significant difference (P > 0.05). The recurrence rates of the VATS wedge resection group and VATS radical resection group were 14.3% and 3.0%, without significant difference (P > 0.05). The authors concluded it is safe, minimally invasive and meaningful to perform VATS wedge resection on the elderly high-risk patients with stage I PNSCLC. Video Assisted Thoracoscopic Surgery (VATS) Sep 15 8 Scientific Rationale Update – July 2014 The Version 4. 2014 National Comprehensive Cancer Network (NCCN) guidelines on non-small cell lung cancer (NSCLC) notes the following: For resection, VATS or minimally invasive surgery should be strongly considered for patients with no anatomic or surgical contraindications, as long as there was no compromise of standard oncologic and dissection principles of thoracic surgery. The choice of biopsy or surgical excision should be based on the clinical suspicion of lung cancer, location of lesion (feasibility of surgical identification and resection by minimally invasive video assisted thoracic surgery (VATS) and patient preference. In patients with suspected NSCLC, many techniques are available for tissue diagnosis. Diagnostic tools that should be routinely available include: Video assisted thoracic surgery (VATS) and open surgical biopsy. In high volume centers with significant VATS experience, VATS lobectomy in selected patients results in improved early outcomes (i.e., decreased pain, reduced hospital length of stay, more rapid return to function, fewer complications) without compromise of cancer outcomes. Patients with occult positive N2 nodes discovered at the time of pulmonary resection should continue with the planned resection along with formal mediastinal lymph node dissection. If N2 disease is noted in patients undergoing VATS the surgeon may consider stopping the procedure so that induction therapy can be administered before surgery; however continuing the procedure is also an option. Video-assisted thoracic surgery (VATS) which is also known as Thoracoscopic lobectomy, is a minimally invasive surgical treatment that is currently being investigated in all aspects of lung cancer. Robotic VATS seems to be more expensive with longer operating times than conventional VATS. Scientific Rationale Update - March 2011 Lung volume reduction surgery (LVRS) or reduction pneumoplasty, also referred to as lung shaving or lung contouring, is performed on patients with severe emphysema in order to allow the remaining compressed lung to expand, and thus, improve respiratory function. The two main surgical procedures for lung volume reduction surgery (LVRS), also known as reduction pneumoplasty include thoracotomy or median sternotomy with bilateral pneumectomy using stapler resection, and endoscopic surgery (thoracoscopy or video-assisted thoracoscopic surgery) with laser ablation and/or stapler resection of diseased lung tissue. The decision about whether to perform LVRS via thoracotomy or thoracoscopy depends in large part on the surgical and institutional expertise. Medicare-covered LVRS approaches are limited to bilateral excision of a damaged lung with stapling performed via median sternotomy or video-assisted thoracoscopic surgery. Mckenna et al (2004) compared lung volume reduction surgery with medical therapy for severe emphysema, including randomized and nonrandomized comparisons of the median sternotomy and video-assisted thoracoscopic approaches for lung volume Video Assisted Thoracoscopic Surgery (VATS) Sep 15 9 reduction surgery. Lung volume reduction surgery was performed by median sternotomy only at 8 centers and video-assisted thoracoscopy only at 3 centers; 6 centers randomized the approach to lung volume reduction surgery. Mortality, morbidity, and functional status were assessed. In the nonrandomized comparison, 359 patients received lung volume reduction surgery by median sternotomy, and 152 patients received lung volume reduction surgery by video-assisted thoracoscopy. The 90-day mortality was 5.9% for median sternotomy and 4.6% for video-assisted thoracoscopy. Overall mortality was 0.08 deaths per person-year for median sternotomy and 0.10 deaths per person-year for video-assisted thoracoscopy. Complication rates were low and not statistically different for the 2 approaches. The median hospital length of stay was longer for median sternotomy than for videoassisted thoracoscopy (10 vs 9 days). By 30 days after surgery, 70.5% of median sternotomy patients and 80.9% of video-assisted thoracoscopy patients were living independently. Functional outcomes were similar for median sternotomy and videoassisted thoracoscopy at 12 and 24 months. Similar results were noted for the randomized comparison. The investigators concluded morbidity and mortality were comparable after lung volume reduction surgery by video-assisted thoracoscopy or median sternotomy, as were functional results. The video-assisted thoracoscopic approach to lung volume reduction surgery allowed earlier recovery than median sternotomy. Roberts et al (1998) compared patients undergoing bilateral lung volume reduction (BLVRS) via median sternotomy and thoracoscopic techniques with emphasis on hospital course and complications. Patients were analyzed for mortality and morbidity, using a combination of prospective data analysis and retrospective chart review. Patients undergoing BLVRS via median sternotomy were older than those undergoing video-assisted thoracoscopic surgery (VATS) procedures (63.9+/-6.89 vs 59.3+/-9.4 years). Operating time was longer for the VATS procedure (147 versus 129 minutes) while estimated blood less was greater for median sternotomy (209 versus 82 L). Significant differences were found in intensive care unit stay, days intubated, life-threatening complications, respiratory complications, requirement for tracheostomy, and death that favored VATS BLVRS. When only later cohorts of patients were compared, more life-threatening complications and deaths were found in patients undergoing BLVRS by median sternotomy. There were no differences between early and late median sternotomy BLVRS patients. Twenty-six percent of the lethal complications in median sternotomy BLVRS patients were bowel perforations, equally divided between duodenal ulcers and colons. The investigators concluded bilateral video-assisted volume reduction offers equivalent functional outcome with potentially decreased morbidity and mortality. Gastrointestinal perforations can complicate the management of these patients. Stammberger et al (1997) investigated the functional results, complications and survival of bilateral VATS lung volume reduction surgery in 42 of 143 candidates with severe, nonbullous pulmonary emphysema. Patients were short of breath on minimal exertion due to severe airflow obstruction and hyperinflation (FEV1 < 30%) pred., TLC > 130% pred., RV > 200% pred.). LVRS was performed bilaterally by VATS using endoscopic staplers without buttressing the staple lines. Pulmonary function test (PFT), MRC dyspnea score and 12 min walking distance were assessed preoperatively, at 3, 6 and 12 months. In addition lung function was measured at hospital discharge. The patients reported a marked relief of dyspnea, which persisted at all follow-up visits. FEV1 increased from 0.80 +/- 0.24 (L) to 1.14 +/0.41 (L) postoperatively, a 43% gain. A relevant increase of FEV1 persisted for at least 1 year. The residual volume to total lung capacity ratio decreased from 0.64 to 0.56 at hospital discharge. The mean 12 min walking distance increased from 500 +/- 195 (m) to 770 +/- 222 (m) after 1 year. The mean hospital stay was 13 +/5.5 days (median 12.0), drainage time was 9 +/- 4.3 (median 8.0) days. There was Video Assisted Thoracoscopic Surgery (VATS) Sep 15 10 no 30 day mortality. Three patients died between 2 and 15 months postoperatively by non surgery related reasons. One patient underwent lung transplantation 5 months after surgical lung volume reduction. The investigators concluded in a selected group of patients with severe, nonbullous pulmonary emphysema, bilateral LVR by VAT results in instantaneous postoperative improvement in pulmonary function and dyspnea. These favorable effects, including an amelioriation in exercise performance, lasted for at least 1 year. Some individuals with severe intractable, disabling hyperhidrosis often remain symptomatic despite conservative medical treatment and thus may require surgical intervention. The goal of surgery for hyperhidrosis is to disrupt the sympathetic supply to sweat glands by destroying relevant ganglions in the upper thoracic sympathetic chain. Although sympathectomy is sometimes performed as an open procedure, most current techniques involve a limited sympathectomy that is performed endoscopically. Wolosker et al (2007) prospectively investigated, how surgery affects patients with palmar hyperhidrosis (PH) and plantar hyperhidrosis (PLH) over one-year period. 70 consecutive patients with combined PH and PLH underwent VATS at the T2, T3, or T4 ganglion level (47 women and 23 men, with mean age of 23 years). Immediately after the operation, all the patients said they were free from PH episodes, except for two patients (2.8%) who suffered from continued PH. Compensatory hyperhidrosis (CH) of various degrees was observed in 58 (90.6%) patients after one year. Only 13 (20.3%) suffered from severe CH. There was a great initial improvement in PLH in 50% of the cases, followed by progressive regression, such that only 23.4% still presented that improvement after one year. The number of cases without overall improvement increased progressively (from 17.1% to 37.5%) and the numbers with slight improvement remained stable (32.9-39.1%). Of the 24 patients with no improvement after one year, 6 patients graded plantar sweating worse. The investigators concluded patients with PH and PLH who undergo VATS to treat their PH present a good initial improvement in PLH that reduces to a lower level of improvement after the one-year period. Prasad et al (2010) performed a prospective study of endoscopic thoracic sympathectomies for palmar hyperhidrosis based on case histories and a prospective pre- and postoperative questionnaire survey. The sample comprised of 322 patients with a mean age of 24 years at a single institution. Bilateral video-assisted thoracoscopic T3 level sympathectomies were performed in all cases. All patients had immediate cessation of palmar hyperhidrosis. The mean postoperative stay was 1.1 days. A questionnaire was completed based on their response to a telephone conversation or e-mail. A paired t test and Wilcoxon test was performed on these data and it showed significant improvement in quality of life. Compensatory sweating was found to be the most troublesome side effect for all patients. It was seen in 63% of the patients. This is similar to other reports of compensatory sweating; however, the figure decreases to 29% if we disregard the percentage of patients who reported only mild compensatory sweating. Rieger et al (2008) performed a retrospective analysis on prospectively collected data of 402 thoracic sympathectomies performed in 204 consecutive patients with palmar-plantar (n=123), palmar-axillary (34), isolated axillary (35), and craniofacial (12) hyperhidrosis. The standard procedure was video-assisted thoracoscopic resection of the sympathetic chain from T2 to T4/5. All procedures were performed thoracoscopically without serious perioperative complications. Postoperative morbidity was 2.5% (10/402) including two cases of incomplete Horner's syndrome (0.5%). One hundred forty-three patients were followed for a mean of 21 months (686). Palmar hyperhidrosis was eliminated in 100% of cases and axillary Video Assisted Thoracoscopic Surgery (VATS) Sep 15 11 hyperhidrosis in 98.5%. There were three axillary recurrences (1.5%). Of all patients, 60% suffered from transient postsympathectomy neuralgia which was mild in the majority of cases. Strong compensatory sweating occurred in 17% of patients with palmar-plantar and palmar-axillary hyperhidrosis and in 53% of patients with isolated axillary hyperhidrosis. In the palmar-plantar and palmar-axillary groups, 92% were very satisfied with the postoperative results, 90% reported increased quality of life, and 93% would repeat the operation. The corresponding numbers in patients with isolated axillary hyperhidrosis were 47%, 44%, and 66%, respectively. The investigators concluded video-assisted thoracoscopic resection of the sympathetic chain from T2 to T4-5 is safe and effective and leads in almost 100% of cases to the elimination of palmar and axillary hyperhidrosis. In contrast to the excellent results in patients with palmar-plantar and palmar-axillary hyperhidrosis, outcome in patients with isolated axillary hyperhidrosis was impaired by a high rate of disturbing compensatory sweating. Scientific Rationale Update – March 2010 Video Assisted Thoracoscopic Surgery (VATS) Video Assisted Thoracoscopic Surgery is also referred to as a thoracoscopic biopsy or VATS. This type of thoracic surgery takes its roots from medical thoracoscopy or pleuroscopy, and has now been technically developed to the point that it can replace thoracotomy in many indications. VATS is performed using a small video camera that is introduced into the patient's chest via a scope. The surgeon is able to view the instruments that are being used along with the anatomy that is being operated on. The camera and instruments are inserted through separate holes in the chest wall also known as "ports". These small ports are advantageous because of the chance for infection and dehiscence are drastically reduced. This allows for a faster recovery by the patient and a greater chance for the wound to heal. Traditionally, thoracic surgery performed for diagnosis or treatment of chest conditions has required access to the chest through thoracotomy or sternotomy incisions. Sternotomy requires the use of a sternal saw to divide the sternum and requires spreading of the divided portions of the sternum with a sternal retractor to allow for visualization of the thoracic structures, passage of instruments into the chest, and removal of specimens. Thoracotomy, as most commonly performed, requires division of one or more major muscles of the chest wall including the latissimus dorsi, pectoralis or serratus muscles, along with spreading of the ribs with a rib spreader. Because the joints of the ribs with the vertebral bodies have only limited flexibility, the use of a rib spreader usually results in rib fractures in the process of rendering the interspace between the ribs wide enough to perform diagnostic or therapeutic maneuvers. Because of this, thoracic surgeons generally intentionally remove a section of one or more ribs in an effort to prevent splintered rib fractures associated with the use of a rib spreader. Sternotomy and thoracotomy have been proven over decades to provide highly effective means of access to thoracic structures and in general are tolerated by patients. However, both incisions have the potential for causing significant pain that may last for extended periods and both result in bony fractures that require a minimum of six weeks to heal during which time patients must refrain from heavy lifting or strenuous activity. The great advantage of VATS over sternotomy or thoracotomy is avoidance of muscle division and bone fractures that allows for diminished duration and intensity of pain and a shorter time to return to full activity. The instrumentation for VATS includes the use of a camera-linked 5mm or 10mm fiberoptic scope, with or without a 30 degree angle of visualization, and either conventional thoracic instruments or laparoscopic instruments. Unlike with Video Assisted Thoracoscopic Surgery (VATS) Sep 15 12 laparoscopy, carbon dioxide insufflation is not generally required with VATS due to the inherent vault-like shape of the thoracic cavity. However, lung deflation on the side of the chest where VATS is being performed is a must to be able to visualize and pass instruments into the thorax; this is usually effected with a double-lumen endotracheal tube that allows for single lung ventilation or a bronchial blocker delivered via a standard single-lumen endotracheal tube. A lung biopsy may be performed using either a closed or an open method. Closed methods are performed through the skin or through the trachea and are also known as transthoracic, or percutaneous biopsies. A transbronchial biopsy is performed through a fiberoptic bronchoscope through the main airways of the lungs. VATS would not be indicated in situations appropriate for closed lung biopsy (i.e. transbronchial, or percutaneous biopsies). Video-assisted thoracoscopic surgery (VATS) may be performed on selected patients in place of open lung biopsy. The surgeon makes several small incisions in the chest wall. A thorascope, a thin, hollow, lighted tube with a tiny video camera mounted on it, is inserted through one of the small incisions. The other incisions allow the surgeon to insert special instruments to retrieve tissue for biopsy. Medical Thoracoscopy Medical thoracoscopy or pleuroscopy refers to the percutaneous insertion of an endoscope into the pleural space. This procedure is used mainly for diagnostic purposes in pleural diseases. The most common indications for pleuroscopy are diagnosis of pleural effusion with inspection of the pleural cavity, combined with biopsies from the parietal and visceral pleura, as well as treatment of malignant or other therapy-refractory effusions by talc pleurodesis. It is a relatively simple and inexpensive technique because it can be performed in an endoscopy room, under local anesthesia or conscious sedation, through a single entry site with nondisposable instruments. Medical pleuroscopy (MP) offers a safe and minimally invasive tool for interventional pulmonologists. It allows diagnosis of unexplained effusion, while at the same time allowing drainage and pleurodesis. It can also help in the diagnosis of diffuse interstitial disease or associated peripheral lung abnormality in the presence of effusion. It can have a therapeutic role in pneumothorax and hyperhidrosis or chronic pancreatic pain. Scientific Rationale Initial Video-assisted thoracoscopic surgery (VATS) is a minimally invasive surgical technique that has been used to replace a traditional thoracotomy. This procedure has been associated with a reduction in surgical morbidity and is particularly useful for those with significant medical comorbidity. Each patient is evaluated individually to determine if VATS is appropriate. The best candidates are those who have never had chest surgery before, because scar tissue from previous procedures can make access into the chest cavity more challenging. VATS generally involves at least three small incisions or access ports placed in any intercostal space. One port is used for the video thoracoscope and two ports for endoscopic instrumentation. Although the access ports are small, the rigid instruments could cause trauma to the intercostal nerves and rib periosteum that can result in regional postoperative discomfort. The size of video-assisted surgical incisions depends on the goals of the procedure and the anatomic findings at the time of exploration. In patients undergoing Video Assisted Thoracoscopic Surgery (VATS) Sep 15 13 anatomic resection, such as segmentectomy or lobectomy, at least one of the incisions is extended to permit extraction of the resected lung from the hemithorax. For most video-assisted thoracic procedures, the operation requires single-lung ventilation. Many patients with chronic respiratory insufficiency and preserved ventilation-perfusion matching, tolerate periods of selective lung ventilation. The ability of many patients with severe emphysema to tolerate selective ventilation has led to the application of thoracoscopy for lung volume reduction surgery. The usefulness of VATS in both diagnostic and staging of lung cancer is widely recognized. Its utilization as an analytical tool in pleural and pulmonary disease is well established especially in evaluating solitary pulmonary nodules, and pulmonary processes of unclear etiology. It allows a complete exploration of the pleural cavity, biopsy of mediastinal node stations not accessible at mediastinoscopy, assessment of extension of primary tumor, and biopsy of associated lung nodules. Video-assisted thoracic surgery has become routine for the removal of solitary pulmonary nodules, 3 cm or less in diameter, of unknown etiology. It is also useful for diagnosing certain pneumonia infections, diagnosing infections, and treating repeatedly collapsing lungs. Doctors are continuing to develop other uses for VATS. (2008) Annals of Thoracic Surgery: Video-assisted thoracoscopic surgery (VATS) lobectomy provides a minimally invasive approach for the management of earlystage lung cancer. Questions about the safety of VATS lobectomy and its adequacy as a cancer operation compared with open thoracotomy have hindered its universal acceptance among thoracic surgeons. Evidence suggests that VATS lobectomy can be safely performed and is an adequate cancer operation for early-stage non-small cell lung cancer. However, additional adequately powered well-balanced studies comparing VATS with open thoracotomy for lobectomy are necessary. Shaw et al. (2008) reviewed data on 180 video-assisted thoracoscopic surgery (VATS) patients who underwent thoracoscopic lobectomy or sublobar anatomic resection between January 2002 and December 2006. Similar variables were evaluated for patients aged older than 80 years, those with a forced expiratory volume in 1 second (FEV1) that was less than 50% predicted, those who had undergone pre-op neoadjuvant therapy, and those who had undergone lung-sparing anatomic resections. Thoracoscopic anatomic lung resection was performed successfully in 166 patients. One of 180 patients (0.6%) died, and 14 patients (9.2%) underwent conversions. Overall median length of stay was 4 days (range, 1 to 98; interquartile range [IQR], 3), and median duration of chest tube drainage was 3 days (range, 0 to 35 days; IQR, 2). The median length of hospital stay and median chest tube duration for the group aged 80 years and older was 5 and 3 days; for the segmental resection group, 4 and 3 days; for the chemotherapy or radiotherapy induction group, 3.5 and 3 days; and for the FEV1 less than 50% group, 5.5 and 4 days, respectively. No patients died in any of these groups. Thoracoscopic lung resection can be performed safely in selected patients aged 80 years and older, in those with marginal pulmonary function, and in those with pathologic response to neoadjuvant therapy. Nicastri et al. (2008) collected data from 153 consecutive patients who underwent (video-assisted thoracoscopic surgery) lobectomy and assessed the perioperative outcomes, postoperative pain, and chemotherapy course. A total of 111 of 127 patients with lung cancer had stage I non-small cell lung cancer. There were 9 major complications (6%), including 1 perioperative death (0.7%). Conversion to thoracotomy occurred in 14 patients (9.2%). Blood transfusion was required in 11 patients (7%). The median chest tube time was 3 days, and the length of hospital Video Assisted Thoracoscopic Surgery (VATS) Sep 15 14 stay was 4 days; 94.4% of patients went home at the time of discharge, and 5.6% of patients required a rehabilitation facility. At a median postsurgical follow-up time of 2 weeks, the mean postoperative pain score was 0.6 (0-3), 73% of patients did not use narcotics for pain control, and 47% of patients did not use any pain medication. Of patients receiving chemotherapy (N = 26), 73% completed a full course on schedule and 85% received all intended cycles. Thoracoscopic (videoassisted thoracic surgery) lobectomy can be performed safely. Discharge independence and low pain estimates in the early postoperative period suggest that this approach may be beneficial. Furthermore, there is a trend toward improved tolerance of chemotherapy. Jones et al. (2008) compared the outcomes of converted VATS patients with open thoracotomy controls. Between May 1992 and April 2006, 30 of 286 VATS lobectomies for lung cancer required intraoperative conversion to open thoracotomy. Four patients were of advanced stage and excluded from the study. The remaining patients were matched 2:1 with open thoracotomy controls by age, sex, cancer stage, year, and type of operation. Postoperative complications and pathology were determined from the hospital discharge summary and pathology report. Long-term survival information was obtained from the family doctor or central registry. There were no statistically significant differences in postoperative complications between the two groups (p = 0.093). There were no in-hospital deaths in the converted VATS group. Conversion during attempted VATS resection does not prejudice short-term or long-term surgical outcomes. We therefore suggest that VATS lobectomy should be the treatment strategy of choice for stage I and II non-small cell lung cancer in view of the well-established short-term benefits and equivalent survival associated with successful VATS resection. Sawada et al. (2008) completed a study that identified 198 patients that had undergone VATS lobectomy for lung cancer between 1998 and 2002. The 5-year survival rate and frequency of recurrence were evaluated as the long-term outcomes, and the frequency of perioperative complications were also evaluated as the short-term outcomes. Median postoperative follow-up period was 72.1 months. Of the 198 patients, 138 and 30 were diagnosed as having p-stage IA and IB disease, respectively, while the remaining 30 patients had more advanced disease. Perioperative complications were observed in 20 patients (10.1%), however, there were no perioperative mortalities. Recurrence was observed in 26 patients (13.1%): of these, 11 patients showed local recurrence, including malignant pleural effusion and mediastinal lymph node recurrence, and 16 patients showed distant metastasis, the lung being the commonest site of metastasis; six patients had both local recurrence and distant metastasis. During the study period, there were 26 deaths (13.1%), of which 17 were due to lung cancer and 9 were due to other causes. The 5-year overall survival rates of the patients with p-stage IA and IB disease were 93.5% and 81.6%, respectively. VATS lobectomy for the treatment of lung cancer is as feasible and safe as open lobectomy in terms of both very long- and short-term outcomes. (2007) American College of Chest Physicians - In patients with stage I non-small cell lung cancer (NSCLC), who are considered appropriate candidates, the use of videoassisted thoracic surgery (VATS) by surgeons experienced in these techniques is an acceptable alternative to open thoracotomy. Grade of recommendation is 1B, which refers to stong recommendation. (2007) A Clinical Trial is currently recruiting patients. The purpose of this study is to examine the feasibility of VATS lobectomy for clinical stage IB or II non-small cell lung cancer. Success is defined as VATS lobectomy without conversion. If success Video Assisted Thoracoscopic Surgery (VATS) Sep 15 15 rate over 90%, VATS lobectomy is considered as feasible procedures for clinical stage IB or II non-small cell lung cancer. Cedar-Sinai Medical Center is the leader in minimally invasive surgery of the lung. McKenna et al. (2006) performed a retrospective review between 1992 and 2004, on 1100 patients who underwent either VATS lobectomy or pneumonectomy for bronchogenic carcinoma. This included 595 (54.1%) women and 505 men (45.9%), with mean age of 71.2 years (range 39-85 years). There were 9 deaths (0.8%)none were intra-operative or due to bleeding. 932 patients had no postoperative complications (84.7%). Blood transfusion was required in 45 of the 1100 patients (4.1%). Length of hospital stay was median of 3 days, mean of 4.78 days. One hundred and eighty (180) patients were discharged on post-operative day (POD) 1 or 2 (20%). Conversion to a thoracotomy occurred in 28 patients (2.5%). In summary, the authors of this study feel that VATS lobectomy with anatomic dissection can be performed with low morbidity and mortality rates. The risk of intraoperative bleeding or recurrence in an incision seems minimal. However, this was not a randomized study and careful monitoring of the surgeons initial VATS outcomes is necessary before adopting this procedure into routine practice. Data from providers using this model and long-term disease free and overall survival outcomes from this series are needed to confirm the success of VATS lobectomy. Shigemura et al. (2006) performed a multi-institutional, retrospective review in 145 patients. Patients with clinical stage IA disease, with tumor size less than or equal to 2 cm in diameter, from three institutions underwent a complete VATS (c-VATS, n = 56), an assisted VATS (a-VATS, n = 34), or a conventional open (open, n = 55) approach for pulmonary lobectomy and lymph node dissection. Patients undergoing lobectomy and lymph node dissection with c-VATS had less blood loss, faster recovery, shorter hospitalization, and longer operating times than did patients undergoing the lobectomy with the a-VATS and open approaches. At a mean followup of 38.8 months, Kaplan-Meier probabilities of survival at 5 years were as follows: c-VATS, 96.7%; a-VATS, 95.2%; open, 97.2%. There was no significant difference in the rate of recurrence among the 3 different procedures. VATS lobectomy, a safe procedure with earlier return to normal activities, can be regarded as an acceptable cancer operation for the patients with peripheral non–small cell lung cancer less than or equal to 2 cm in diameter (clinical stage IA) with the same long-term survivals as open surgery. Petersen et al. (2006) conducted a retrospective study of 97 patients, from 1996 to 2005, with NSCLC who underwent lobectomy after induction therapy to determine the feasibility of thoracoscopic lobectomy compared with conventional thoracotomy lobectomy. Lobectomy was performed by thoracotomy in 85 patients and thoracoscopically in 12 patients (1 conversion), with complete resection in all patients. All patients received induction chemotherapy, and 74 (76%) received induction radiotherapy as well: 66 of 85 (78%) in the thoracotomy group and 8 of 12 (67%) in the thoracoscopy group. The overall median survival was 2.3 years, with no difference between the groups. Patients undergoing a thoracoscopic lobectomy had a shorter median hospital stay (3.5 vs 5 days, p = 0.0024) and chest tube duration (2 vs 4 days, p < 0.001). There were no significant differences in 30-day mortality, hemorrhage, pneumonia, or respiratory failure. Thoracoscopic lobectomy is a feasible approach for selected patients undergoing resection after induction therapy, and is associated with shorter hospital stay and chest tube duration. Long-term follow-up of survival will determine the role of thoracoscopic lobectomy in the management of patients after induction therapy. Video Assisted Thoracoscopic Surgery (VATS) Sep 15 16 (2005) Scottish Intercollegiate Guidelines Network (SIGN). Video-assisted thoracoscopic surgery (VATS) resection, undertaken by an appropriately skilled surgeon, may be offered to selected patients with clinical stage I lung cancer. Ohtsuka et al. (2004) completed a study of 106 patients with lung cancer in whom major pulmonary resection by VATS, between August 1999 and March 2003, was attempted. Their clinical stage I disease was determined preoperatively. The number of procedures that were converted to open thoracotomy and the reasons for conversion, the intraoperative blood loss, interval between surgery and chest tube removal, length of postoperative hospital stay, postoperative complications, mortality rate, prognoses, and patterns of recurrence, were all evaluated. VATS was completed in 95 patients, whereas in another 11 patients (10%) conversion to open thoracotomy was required. The operative procedures were lobectomy in 86 patients, segmentectomy in 8 patients, and bilobectomy in 1 patient. In 95 patients VATS, postoperative complications developed in 9 patients (9%), and 1 patient (1%) died from pneumonia. In the 86 patients without complications, the mean postoperative hospital stay was 7.6 days (range, 4 to 15 days). In a mean follow-up period of 25 months (range, 6 to 48 months) in patients with non-small cell lung cancer (NSCLC), including the one perioperative death, the 3-year survival rate was 93% in 82 patients with clinical stage I disease, and 97% in 68 patients with pathologic stage I disease. The 3-year disease-free survival rate was 79% in patients with clinical stage I disease, and 89% in patients with pathologic stage I disease. Local recurrence was observed in six patients (6%): recurrence in mediastinal lymph nodes in five patients, and in the bronchial stump in one patient. Major pulmonary resection by VATS is acceptable in view of its low perioperative mortality and morbidity, and is an adequate procedure for the achievement of local control and good prognosis in patients with clinical stage I NSCLC, although long-term follow- up and prospective studies are required. The biggest concerns regarding VATS lobectomy center on risk and management of intraoperative bleeding, tumor recurrence in the incision, and the adequacy of the cancer operation. The controversies with VATS involve the following issues: anatomic dissection versus mass ligation of hilar structures, the length of the utility incision, the use of a rib spreader, the use of endoscopic instruments versus conventional instruments, and visualization through the incision or only on the monitor. The least controversial issue is how hilar structures are addressed during a VATS lobectomy. Although a simultaneous individual stapling lobectomy with mass stapling of the vessels and the fissure was reported, this technique should be discouraged. Individual ligation of vessels and bronchus has been the standard for more than 50 years, and so it should remain. An operation should not be compromised to make it a minimally invasive operation. Although the public perceives video-assisted thoracoscopic (VATS) surgery as advantageous because it is less invasive, the medical community has questioned the adequacy and safety of VATS lobectomy. Clinical studies have not addressed these issues because they had only short-term follow up. Indications for open thoracotomy include chest wall invasion, complex procedures (such as sleeve resections), and prior chemotherapy or radiation. Limited resections should be avoided for tumors >3 cm in size whenever possible. Defining the role of VATS will require randomized, prospective trials to demonstrate efficacy equivalent to a traditional surgical approach. As noted above, the gold standard in chest surgery continues to be the thoracotomy. Only 5% of lobectomies in the U.S. are performed by VATS. Some of the thoracic societies including the Annals of Thoracic Surgery, feel that well-balanced studies Video Assisted Thoracoscopic Surgery (VATS) Sep 15 17 comparing VATS with open thoracotomy for lobectomy are lacking in the literature. Others, such as the American College of Chest Physicians and the Scottish Intercollegiate Guidelines Network, feel that video-assisted thoracoscopic surgery (VATS) resection, undertaken by an appropriately skilled surgeon, may be offered to selected patients with clinical stage I non-small cell lung cancer. Review History August 2008 March 2010 March 2011 January 2012 January 2013 January 2014 July 2014 September 2014 September 2015 Medical Advisory Council Initial Approval. Health Net, Inc. considers video-assisted thoracoscopic surgery (VATS) medically necessary for the following scenarios: to remove a solitary pulmonary nodule, 3 cm or less in diameter; or lobectomy for stage I non-small cell lung cancer. Revised policy to include VATS as medically necessary as an alternative to Open Lung Biopsy, where open lung biopsy is clinically indicated. Revised title to Video-Assisted Thoracoscopic Surgery (VATS). Revised policy to included VATS as medically necessary for the treatment of intractable hyperhidrosis and as an alternative to thoracotomy for lung volume reduction surgery when criteria is met. Medicare table added. Code Updates Update – no revision Update – no revision. Codes updated. Update – no revision. Codes updated. Update – no revision. Codes updated. Update – revised policy to consider VATs medically necessary for diagnostic and therapeutic pleural, lung, and mediastinal surgery. Code updates Update – no revisions This policy is based on the following evidence-based guidelines: 1. 2. 3. Scott WJ, Howington J, Feigenberg S, et al. American College of Chest Physicians. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007 Sep;132(3 Suppl):234S-42S. Scottish Intercollegiate Guidelines Network (SIGN). Management of patients with lung cancer. A national clinical guideline. Edinburgh (Scotland): Scottish Intercollegiate Guidelines Network (SIGN); 2005 Feb. 63 p. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer. Version 4.2014. Update 7.2015 References – Update September 2015 1. 2. 3. 4. Cai HB, Li YX, Li Z. Short term curative effect of video assisted thoracoscopic lobectomy for early-stage lung cancer. Indian J Cancer. 2015 Feb;51 Suppl 2:e37-41. Gill RR, Zheng Y, Barlow JS et al. Image-guided video assisted thoracoscopic surgery (iVATS) - phase I-II clinical trial. J Surg Oncol. 2015 Jul;112(1):18-25. Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensitymatched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg. 2015 Apr 26. Hamaji M, Chen F, Matsuo Y, et al. Video-assisted thoracoscopic lobectomy versus stereotactic radiotherapy for stage I lung cancer. Ann Thorac Surg. 2015 Apr;99(4):1122-9. Video Assisted Thoracoscopic Surgery (VATS) Sep 15 18 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. Haruki T, Miwa K, Araki K, et al. Distribution and Prevalence of Locoregional Recurrence after Video-Assisted Thoracoscopic Surgery for Primary Lung Cancer. Thorac Cardiovasc Surg. 2015 Aug 10. Imperatori A, Rotolo N, Spagnoletti M, et al. Risk factors for postoperative recurrence of spontaneous pneumothorax treated by video-assisted thoracoscopic surgery. Interact Cardiovasc Thorac Surg. 2015 May;20(5):64751; discussion 651-2. Laursen LØ, Petersen RH, Hansen HJ, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur J Cardiothorac Surg. 2015 Jun 18. Liu C, Li Z, Bai C, et al. Video-assisted thoracoscopic surgery and thoracotomy during lobectomy for clinical stage I non-small-cell lung cancer have equivalent oncological outcomes: A single-center experience of 212 consecutive resections. Oncol Lett. 2015 Mar;9(3):1364-1372 Liu Z, Yang J, Lin L, Huang J, Jiang G. Unilateral video-assisted thoracoscopic extended thymectomy offers long-term outcomes equivalent to that of the bilateral approach in the treatment of non-thymomatous myasthenia gravis. Interact Cardiovasc Thorac Surg. 2015 Aug 7 Maniscalco P, Tamburini N, Quarantotto F, et al. Long-term outcome for early stage thymoma: comparison between thoracoscopic and open approaches. Thorac Cardiovasc Surg. 2015 Apr;63(3):201-5. Murakawa T, Ichinose J, Hino H, et al. Long-term outcomes of open and videoassisted thoracoscopic lung lobectomy for the treatment of early stage non-small cell lung cancer are similar: a propensity-matched study. World J Surg. 2015 May;39(5):1084-91. Nakano T, Endo S, Endo T, et al. Surgical Outcome of Video-Assisted Thoracoscopic Surgery vs. Thoracotomy for Primary Lung Cancer >5 cm in Diameter. Ann Thorac Cardiovasc Surg. 2015 May 25 Numan RC, Baas P, Klomp HM, Wouters MW. Optimal surgical management of pulmonary metastases: VATS versus thoracotomy. Respirology. 2015 Aug 9. Radkani P, Joshi D, Barot T, Williams RF. Robotic video-assisted thoracoscopic lung resection for lung tumors: a community tertiary care center experience over four years. Surg Endosc. 2015 Jun 20. Raposio E, Caruana G. Video-assisted Thoracic Sympathicotomy for the Treatment of Palmar and Axillary Hyperhidrosis: A 17-Year Experience. Surg Laparosc Endosc Percutan Tech. 2015 Feb 26. Reichert M, Steiner D, Kerber S,et al. A standardized technique of systematic mediastinal lymph node dissection by video-assisted thoracoscopic surgery (VATS) leads to a high rate of nodal upstaging in early-stage non-small cell lung cancer. Surg Endosc. 2015 Jul 14. Tane S, Nishio W, Okuma H, et al. Operative outcomes of thoracoscopic lobectomy for non-small-cell lung cancer. Asian Cardiovasc Thorac Ann. 2015 Jul 22. Wu N, Wu L, Qiu C, et al. A comparison of video-assisted thoracoscopic surgery with open thoracotomy for the management of chest trauma: a systematic review and meta-analysis. World J Surg. 2015 Apr;39(4):940-52. Yang B, Liu Y, Dai W, et al. Effects and reasons of conversion during videoassisted thoracic surgery lobectomy. Zhonghua Yi Xue Za Zhi. 2014 Dec 23;94(47):3748-50. Zhang R, Ferguson MK. Video-Assisted versus Open Lobectomy in Patients with Compromised Lung Function: A Literature Review and Meta-Analysis. PLoS One. 2015 Jul 6;10(7):e0124512. Video Assisted Thoracoscopic Surgery (VATS) Sep 15 19 22. Zhou H, Tapias LF, Gaissert HA, et al. Lymph Node Assessment and Impact on Survival in Video-Assisted Thoracoscopic Lobectomy or Segmentectomy. Ann Thorac Surg. 2015 Jul 10. References – Update September 2014 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. Boffa DJ, Dhamija A, Kosinski AS, et al. Fewer complications result from a video-assisted approach to anatomic resection of clinical stage I lung cancer. J Thorac Cardiovasc Surg. 2014 Aug;148(2):637-43. Bryant AS, Cerfolio RJ. Satisfaction and compensatory hyperhidrosis rates 5 years and longer after video-assisted thoracoscopic sympathotomy for hyperhidrosis. J Thorac Cardiovasc Surg. 2014 Apr;147(4):1160-1163.e1. Cai YX, Fu XN, Xu QZ, et al. Thoracoscopic lobectomy versus open lobectomy in stage I non-small cell lung cancer: a meta-analysis. PLoS One. 2013 Dec 31;8(12):e82366. Chen JF, Tu YR, Li X, et al. Video-assisted thoracoscopic extended thymectomy for myasthenia gravis: report of 62 cases. Zhonghua Yi Xue Za Zhi. 2010 Oct 26;90(39):2770-2. Eckardt J, Licht PB. Thoracoscopic or open surgery for pulmonary metastasectomy: an observer blinded study. Ann Thorac Surg. 2014 Aug;98(2):466-70. Gu ZT, Mao T, Chen WH, Fang W. Comparison of Video-assisted Thoracoscopic Surgery and Median Sternotomy Approaches for Thymic Tumor Resections at a Single Institution. Surg Laparosc Endosc Percutan Tech. 2014 Apr 12. He Z, Zhu Q, Wen W, et al. Surgical approaches for stage I and II thymomaassociated myasthenia gravis: feasibility of complete video-assisted thoracoscopic surgery (VATS) thymectomy in comparison with trans-sternal resection. J Biomed Res. 2013 Jan;27(1):62-70. Higuchi M, Yaginuma H, Yonechi A, et al. Long-term outcomes after videoassisted thoracic surgery (VATS) lobectomy versus lobectomy via open thoracotomy for clinical stage IA non-small cell lung cancer. J Cardiothorac Surg. 2014 May 17;9(1):88. Jarrar D. Video-Assisted Thoracoscopy . Medscape. Dec 2013. Available at: http://emedicine.medscape.com/article/1970013-overview Jeon JH, Kang CH, Kim HS, et al. Video-assisted thoracoscopic lobectomy in non-small-cell lung cancer patients with chronic obstructive pulmonary disease is associated with lower pulmonary complications than open lobectomy: a propensity score-matched analysis. Eur J Cardiothorac Surg. 2014 Apr;45(4):640-5. Jurado J, Javidfar J, Newmark A, et al. Minimally invasive thymectomy and open thymectomy: outcome analysis of 263 patients. Ann Thorac Surg. 2012 Sep;94(3):974-81. Jung HS, Kim DK, Lee GD, et al. Video-assisted thoracic surgery for bronchogenic cysts: is this the surgical approach of choice? Interact Cardiovasc Thorac Surg. 2014 Jul 19. Liu CW, Luo M, Mei JD, et al. Perioperative and long-term outcome of thymectomy for myasthenia gravis: comparison of surgical approaches and prognostic analysis. Chin Med J (Engl). 2013 Jan;126(1):34-40. Lin L, Hu D, Zhong C, Zhao H. Safety and efficacy of thoracoscopic wedge resection for elderly high-risk patients with stage I peripheral non-small-cell lung cancer. J Cardiothorac Surg. 2013 Dec 21;8:231. Kim AW, Fonseca AL, Boffa DJ, et al. Experience with thoracoscopic pneumonectomies at a single institution. Innovations (Phila). 2014 MarApr;9(2):82-6. Manoly I, Whistance RN, Sreekumar R, et al. Early and mid-term outcomes of trans-sternal and video-assisted thoracoscopic surgery for thymoma. Eur J Cardiothorac Surg. 2014 Jun;45(6):e187-93. Video Assisted Thoracoscopic Surgery (VATS) Sep 15 20 17. Mason: Murray and Nadel's Textbook of Respiratory Medicine, 5th ed. 18. Morris D, Zamvar V. The efficacy of video-assisted thoracoscopic surgery lung biopsies in patients with Interstitial Lung Disease: a retrospective study of 66 patients. J Cardiothorac Surg. 2014 Mar 10;9:45. 19. Nagai S, Imanishi N, Matsuoka T, et al. Video-assisted thoracoscopic pneumonectomy: retrospective outcome analysis of 47 consecutive patients. Ann Thorac Surg. 2014 Jun;97(6):1908-13. doi: 10.1016/j.athoracsur.2014.02.022. Epub 2014 Mar 28. 20. Ren M, Meng Q, Zhou W, et al. Comparison of short-term effect of thoracoscopic segmentectomy and thoracoscopic lobectomy for the solitary pulmonary nodule and early-stage lung cancer. Onco Targets Ther. 2014 Jul 24;7:1343-7. 21. Rizk NP, Ghanie A, Hsu M, et al. A Prospective Trial Comparing Pain and Quality of Life Measures After Anatomic Lung Resection Using Thoracoscopy or Thoracotomy. Ann Thorac Surg. 2014 Jul 30. 22. Sakamaki Y, Oda T, Kanazawa G, et al. Intermediate-term oncologic outcomes after video-assisted thoracoscopic thymectomy for early-stage thymoma. J Thorac Cardiovasc Surg. 2014 Jan 25. 23. Smith CB, Kale M, Mhango G, et al. Comparative outcomes of elderly stage I lung cancer patients treated with segmentectomy via video-assisted thoracoscopic surgery versus open resection. J Thorac Oncol. 2014 Mar;9(3):383-9. 24. Srisomboon C, Koizumi K, Haraguchi S, et al. Complete video-assisted thoracoscopic surgery for lung cancer in 400 patients. Asian Cardiovasc Thorac Ann. 2013 Dec;21(6):700-7. 25. Teh E, Abah U, Church D, et al. What is the extent of the advantage of videoassisted thoracoscopic surgical resection over thoracotomy in terms of delivery of adjuvant chemotherapy following non-small-cell lung cancer resection? Interact Cardiovasc Thorac Surg. 2014 Jul 11. 26. Wang W, Yin W, Shao W, et al. Comparative study of systematic thoracoscopic lymphadenectomy and conventional thoracotomy in resectable non-small cell lung cancer. J Thorac Dis. 2014 Jan;6(1):45-51 27. Yang J, Xia Y, Yang Y, et al. Risk factors for major adverse events of videoassisted thoracic surgery lobectomy for lung cancer. Int J Med Sci. 2014 Jun 11;11(9):863-9. 28. Yuan ZY, Cheng GY, Sun KL, et al. Comparative study of video-assisted thoracic surgery versus open thymectomy for thymoma in one single center. J Thorac Dis. 2014 Jun;6(6):726-33. 29. Zhang Z, Feng H, Wang X, et al. Can lymph node evaluation be performed well by video-assisted thoracic surgery? J Cancer Res Clin Oncol. 2014 Aug 2. References – Update July 2014 1. Shimada J. The OIDE hook: a retractor for video-assisted thoracic surgery. J Thorac Cardiovasc Surg - 01-APR-2013; 145(4): 1139-40. References – Update January 2014 1. Demmy T, Dexter E. Overview of video assisted thoracoscopic surgery (VATS). UpToDate. May 21, 2013. References – Update January 2013 1. 2. Canale & Beaty: Campbell's Operative Orthopaedics, 12th ed. Surgical Approaches. 2012 Mosby, An Imprint of Elsevier. Ferri: Ferri's Clinical Advisor 2013, 1st ed. Pleural effusion, malignant. 2012 Mosby, An Imprint of Elsevier References – Update January 2012 Video Assisted Thoracoscopic Surgery (VATS) Sep 15 21 1. 2. 3. 4. 5. 6. Bu L, Li Y, Yang F, et al. A retrospective comparative study of the safety, completeness and efficacy of video-assisted thoracoscopic lobectomy versus open lobectomy for non-small-cell lung cancer patients whose tumor size was greater than 5 cm. Beijing Da Xue Xue Bao. 2011 Dec 18;43(6):866-72. Flores RM, Ihekweazu U, Dycoco J, et al. Video-assisted thoracoscopic surgery (VATS) lobectomy: Catastrophic intraoperative complications. J Thorac Cardiovasc Surg. 2011 Dec;142(6):1412-7. Huang W, Wang WR, Deng B, et al. .Several clinical interests regarding lung volume reduction surgery for severe emphysema: meta-analysis and systematic review of randomized controlled trials. J Cardiothorac Surg. 2011 Nov 10;6:148. Kuo E, Bharat A, Bontumasi N, Sanchez C, et al. Impact of Video-Assisted Thoracoscopic Surgery on Benign Resections for Solitary Pulmonary Nodules. Ann Thorac Surg. 2011 Nov 8. Vanderhelst E, De Keukeleire T, Verbanck S, et al. Quality of life and patient satisfaction after video-assisted thoracic sympathicolysis for essential hyperhidrosis: a follow-up of 138 patients. J Laparoendosc Adv Surg Tech A. 2011 Dec;21(10):905-9. Epub 2011 Oct 19. Zhou WY, Chen XF, Zhang L, et al. Video-assisted thoracic surgery lobectomy versus open lobectomy for mini pathologic N2 non-small cell lung cancer. Zhonghua Wai Ke Za Zhi. 2011 Sep 1;49(9):820-4 References - Update March 2011 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. Chen YB, Ye W, Yang WT, et al. Uniportal versus biportal video-assisted thoracoscopic sympathectomy for palmar hyperhidrosis. Chin Med J (Engl). 2009 Jul 5;122(13):1525-8. De Giacomo T, Venuta F, Rendina EA, et al. Video-assisted thoracoscopic treatment of giant bullae associated with emphysema. Eur J Cardiothorac Surg. 1999 Jun;15(6):753-6; discussion 756-7. Divisi D, Battaglia C, Di Francescantonio W, et al. Giant bullous emphysema resection by VATS. Analysis of laser and stapler techniques. Eur J Cardiothorac Surg. 2002 Dec;22(6):990-4. Geiser T, Schwizer B, Krueger T, et al. Outcome after unilateral lung volume reduction surgery in patients with severe emphysema. Eur J Cardiothorac Surg. 2001 Oct;20(4):674-8. He J, Yang Y, Lee Y, et al. Thoracoscopic lung reduction surgery for emphysema. Zhonghua Wai Ke Za Zhi. 1998 May;36(5):299-301 Ishida T, Kohdono S, Fukuyama Y, et al. Video-assisted thoracoscopic surgery of bullous and bleb disorders of the lung using endoscopic stapling device. Surg Laparosc Endosc. 1995 Oct;5(5):349-53. Ishy A, de Campos JR, Wolosker N, et al. Objective evaluation of patients with palmar hyperhidrosis submitted to two levels of sympathectomy: T3 and T4. Interact Cardiovasc Thorac Surg. 2011 Jan 13 Kaiser D, Ennker IC, Hartz C. Video-assisted thoracoscopic surgery--indications, results, complications, and contraindications. Thorac Cardiovasc Surg. 1993 Dec;41(6):330-4. Koebe HG, Kugler C, Dienemann H. Evidence-based medicine: lung volume reduction surgery (LVRS). Cardiovasc Surg. 2002 Oct;50(5):315-22 Kotloff RM, Tino G, Bavaria JE, et al. Bilateral lung volume reduction surgery for advanced emphysema. A comparison of median sternotomy and thoracoscopic approaches. Chest. 1996 Dec;110(6):1399-406. Lin KC, Luh SP. Video-assisted thoracoscopic surgery in the treatment of patients with bullous emphysema. Int J Gen Med. 2010 Aug 30;3:215-20. Liu J, Varoli F. Severe emphysema treated by lung volume reduction surgery. Zhonghua Wai Ke Za Zhi. 1999 Jan;37(1):32-4. Video Assisted Thoracoscopic Surgery (VATS) Sep 15 22 14. Leão LE, de Oliveira R, Szulc R, et al. Role of video-assisted thoracoscopic sympathectomy in the treatment of primary hyperhidrosis. Sao Paulo Med J. 2003 Sep 1;121(5):191-7 15. McKenna RJ Jr, Benditt JO, DeCamp M, et al. Safety and efficacy of median sternotomy versus video-assisted thoracic surgery for lung volume reduction surgery. J Thorac Cardiovasc Surg. 2004 May;127(5):1350-60 16. Naunheim KS, Keller CA, Krucylak PE, et al. 16.Unilateral video-assisted thoracic surgical lung reduction. Ann Thorac Surg. 1996 Apr;61(4):1092-8. 17. Prasad A, Ali M, Kaul S. Endoscopic thoracic sympathectomy for primary palmar hyperidrosis. Surg Endosc. 2010 Aug;24(8):1952-7 18. Rieger R, Pedevilla S, Pöchlauer S. Treatment of palmar and axillary hyperhidrosis: thoracoscopic resection of the sympathetic chain. Chirurg. 2008 Dec;79(12):1151-61 19. Roberts JR, Bavaria JE, Wahl P, et al. Comparison of open and thoracoscopic bilateral volume reduction surgery: complications analysis. Ann Thorac Surg. 1998 Nov;66(5):1759-65. 20. Soon SY, Saidi G, Ong ML, et al. Sequential VATS lung volume reduction surgery: prolongation of benefits derived after the initial operation. Eur J Cardiothorac Surg. 2003 Jul;24(1):149-53. 21. Stammberger U, Thurnheer R, Bloch KE, et al. Bilateral video-assisted thoracoscopic volume reduction surgery for treatment of advanced pulmonary emphysema. Langenbecks Arch Chir Suppl Kongressbd. 1997;114:1283-6. 22. Sun GZ, Xu LH, Zhou B. The choice of thoracoscopic sympathecotomy in the treatment of palmar hyperhidrosis among different procedures. Zhonghua Yi Xue Za Zhi. 2010 Aug 3;90(29):2065-7 23. Tu YR, Li X, Lin M, et al. Video-assisted thoracoscopic sympathectomy for the treatment of palmar hyperhidrosis in 588 cases. Zhonghua Wai Ke Za Zhi. 2007 Nov 15;45(22):1527-9 24. Wang X, Yu FL, Wu ZS, Chen MJ. Clinical application of video-assisted thoracoscopic surgery. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2006 Apr;31(2):284-7. 25. Weder W, Tutic M, Lardinois D, et al. Persistent benefit from lung volume reduction surgery in patients with homogeneous emphysema. Ann Thorac Surg. 2009 Jan;87(1):229-36 26. Wolosker N, Yazbek G, Milanez de Campos JR, et al. Evaluation of plantar hyperhidrosis in patients undergoing video-assisted thoracoscopic sympathectomy. Clin Auton Res. 2007 Jun;17(3):172-6 References Update March 2010 1. 2. Medford ARL, Bennett JA, Free CM, et al. Current Status of Medical Pleuroscopy. Clinics in Chest Medicine - Volume 31, Issue 1 (March 2010). Sheski FD. An overview of medical thoracoscopy. UpToDate. 2008. References Initial 1. Willekes L, Boutros C, Goldfarb M. VATS intraoperative tattooing to facilitate solitary pulmonary nodule resection. Journal of Cardiothoracic Surgery 2008, 3:13doi:10.1186/1749-8090-3-13. 2. Shaw JP, Dembitzer FR, Wisnivesky JP, et al. Video-assisted thoracoscopic lobectomy: state of the art and future directions. Ann Thorac Surg. 2008 Feb;85(2):S705-9. 3. Nicastri DG, Wisnivesky JP, Litle VR, et al. Thoracoscopic lobectomy: report on safety, discharge independence, pain, and chemotherapy tolerance. J Thorac Cardiovasc Surg. 2008 Mar;135(3):642-7. 4. Jones RO, Casali G, Walker WS. Does failed video-assisted lobectomy for lung cancer prejudice immediate and long-term outcomes? Ann Thorac Surg. 2008 Jul;86(1):235-9. Video Assisted Thoracoscopic Surgery (VATS) Sep 15 23 5. Sawada S, Komori E, Yamashita M. Very long-term outcomes of video-assisted thoracoscopic surgery for lung cancer. Surg Endosc. 2008 Jul 12. 6. Flores RM, Alam N. Video-Assisted Thoracic Surgery Lobectomy (VATS), Open Thoracotomy, and the Robot for Lung Cancer. Ann Thorac Surg 2008;85:S710S715. doi:10.1016/j.athoracsur. 2007.09.055. 7. Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. TI Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. SO J Clin Oncol. 2007 Nov 1;25(31):4993-7. 8. Clinical Trials.gov. VATS Lobectomy for Clinical Stage IB or II Lung Cancer. Available at: http://clinicaltrials.gov/ct2/show/NCT00425022 9. Ng T, Ryder BA. Evolution to Video-Assisted Thoracic Surgery Lobectomy after Training: Initial Results of the First 30 Patients. Journal of the American College of Surgeons - Volume 203, Issue 4 (October 2006). 10. Boaron M, Kawamukai K, Parri SF, et al. Surgical procedures in mediastinal lung cancer staging. Annals of Oncology 17 (Supplement 2): ii22–ii23, 2006. doi:10.1093/annonc/mdj914 11. McKenna RJ Jr, Houck W, Fuller CB. TI Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. SO Ann Thorac Surg. 2006 Feb;81(2):421-5; discussion 425-6. 12. Petersen RP, MD, Pham D, Toloza EM, et al. Thoracoscopic Lobectomy: A Safe and Effective Strategy for Patients Receiving Induction Therapy for Non-Small Cell Lung Cancer. Ann Thorac Surg 2006;82:214-219. 13. Shigemura N, Akashi A, Funaki S, et al. Long-term outcomes after a variety of video-assisted thoracoscopic lobectomy approaches for clinical stage IA lung cancer: A multi-institutional study. Thorac Cardiovasc Surg 2006;132:507-512. 14. Loddenkemper R: Medical thoracoscopy—historical perspective. Interventional Pulmonary Medicine, New York: Marcel Dekker; 2004:411-429. 15. Ohtsuka T, Nomori H, Horio H, et al. Is Major Pulmonary Resection by VideoAssisted Thoracic Surgery an Adequate Procedure in Clinical Stage I Lung Cancer. Chest 2004; 125; 1742-1746 DOI 10.1378/chest.125.5.1742. Official Publication of Chest Physicians. 16. Roviaro G, MD, Varoli F, Vergani C, et al. Long-term Survival After Videothoracoscopic Lobectomy for Stage I Lung Cancer.* Chest. 2004;126:725732. 17. Ernst A, Silvestri GA, Johnstone D: Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest 2003; 123:1693-1717. 18. Detterbeck FC, DeCamp MM Jr, Kohman LJ, et al. American College of zChest Physicians. Invasive staging. The guidelines. Chest 2003; 123: 167S–175S. 19. Hoffmann H. Invasive staging of lung cancer by mediastinoscopy and video assisted thoracoscopy. Lung Cancer 2001; 34: S3–5. 20. Lee P, Lan RS, Colt HG: Survey of pulmonologists' perspectives on thoracoscopy. J Bronchol 2003; 10:99-106. 21. Loddenkemper R: Thoracoscopy: What are the perspectives for pulmonologists? J Bronchol 2003; 10:95-96. 22. Loddenkemper R: Medical thoracoscopy. Textbook of Pleural Diseases, London: Arnold; 2003:498-512. 23. Li WW, Lee RL, Lee TW: The impact of thoracic surgical access on early shoulder function: Video-assisted thoracic surgery versus posterolateral thoracotomy. Eur J Cardiothorac Surg 2003; 23:390-396. 24. Ernst A, Hersh CP, Herth F, et al: A novel instrument for the evaluation of the pleural space: An experience in 34 patients. Chest 2002; 122:1530-1534. 25. Video-Assisted Thoracoscopic Surgery (VATS). Mayo Clinic. Available at: http://www.mayoclinic.org/video-assisted-thoracic-surgery/ Video Assisted Thoracoscopic Surgery (VATS) Sep 15 24 26. Video-Assisted Thoracoscopic Surgery. Harvard Medical School. Available at: http://www.health.harvard.edu/diagnostic-tests/video-assisted-thoracicsurgery.htm 27. Poretti FP, Brunner E, Vorwerk D: Simple localization of peripheral pulmonary nodules – CT-guided percutaneous hook-wire localization. Rofo 2002, 174(2):202-7. 28. Roviaro GC, Varoli F, Vergani C, et al. State of the art in thoracopic surgery: a personal experience of 2000 videothoracoscopic procedures and an overview of the literature. Surg Endosc 2002; 16(6): 881–892. 29. Cho K, Ozawa S, Kuzihara M, et al: Cardiorespiratory changes in thoracoscopy under local anesthesia. J Bronchol 2000; 7:215-220. 30. Loddenkemper R: Thoracoscopy under local anesthesia. Is it safe? J Bronchol 2000; 7:207-209. 31. Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest 1999, 115(2):563-8. 32. McKenna Jr RJ: Video-assisted thoracic surgery (VATS) lobectomy for bronchogenic carcinoma. Semin Thorac Cardiovasc Surg 1998; 10:321-325. 33. Naruke T, Asamura H, Kondo H, et al. Thoracoscopy for staging of lung cancer. Ann Thorac Surg 1993; 56: 661–663. Important Notice General Purpose. Health Net's National Medical Policies (the "Policies") are developed to assist Health Net in administering plan benefits and determining whether a particular procedure, drug, service or supply is medically necessary. The Policies are based upon a review of the available clinical information including clinical outcome studies in the peer-reviewed published medical literature, regulatory status of the drug or device, evidence-based guidelines of governmental bodies, and evidence-based guidelines and positions of select national health professional organizations. Coverage determinations are made on a case-by-case basis and are subject to all of the terms, conditions, limitations, and exclusions of the member's contract, including medical necessity requirements. Health Net may use the Policies to determine whether under the facts and circumstances of a particular case, the proposed procedure, drug, service or supply is medically necessary. The conclusion that a procedure, drug, service or supply is medically necessary does not constitute coverage. The member's contract defines which procedure, drug, service or supply is covered, excluded, limited, or subject to dollar caps. The policy provides for clearly written, reasonable and current criteria that have been approved by Health Net’s National Medical Advisory Council (MAC). The clinical criteria and medical policies provide guidelines for determining the medical necessity criteria for specific procedures, equipment, and services. In order to be eligible, all services must be medically necessary and otherwise defined in the member's benefits contract as described this "Important Notice" disclaimer. In all cases, final benefit determinations are based on the applicable contract language. To the extent there are any conflicts between medical policy guidelines and applicable contract language, the contract language prevails. Medical policy is not intended to override the policy that defines the member’s benefits, nor is it intended to dictate to providers how to practice medicine. Policy Effective Date and Defined Terms. The date of posting is not the effective date of the Policy. The Policy is effective as of the date determined by Health Net. All policies are subject to applicable legal and regulatory mandates and requirements for prior notification. If there is a discrepancy between the policy effective date and legal mandates and regulatory requirements, the requirements of law and regulation shall govern. * In some states, prior notice or posting on the website is required before a policy is deemed effective. For information regarding the effective dates of Policies, contact your provider representative. The Policies do not include definitions. All terms are defined by Health Net. For information regarding the definitions of terms used in the Policies, contact your provider representative. Policy Amendment without Notice. Health Net reserves the right to amend the Policies without notice to providers or Members. states, prior notice or website posting is required before an amendment is deemed effective. In some No Medical Advice. The Policies do not constitute medical advice. Health Net does not provide or recommend treatment to members. Members should consult with their treating physician in connection with diagnosis and treatment decisions. No Authorization or Guarantee of Coverage. Video Assisted Thoracoscopic Surgery (VATS) Sep 15 25 The Policies do not constitute authorization or guarantee of coverage of particular procedure, drug, service or supply. Members and providers should refer to the Member contract to determine if exclusions, limitations, and dollar caps apply to a particular procedure, drug, service or supply. Policy Limitation: Member’s Contract Controls Coverage Determinations. Statutory Notice to Members: The materials provided to you are guidelines used by this plan to authorize, modify, or deny care for persons with similar illnesses or conditions. Specific care and treatment may vary depending on individual need and the benefits covered under your contract. The determination of coverage for a particular procedure, drug, service or supply is not based upon the Policies, but rather is subject to the facts of the individual clinical case, terms and conditions of the member’s contract, and requirements of applicable laws and regulations. The contract language contains specific terms and conditions, including pre-existing conditions, limitations, exclusions, benefit maximums, eligibility, and other relevant terms and conditions of coverage. In the event the Member’s contract (also known as the benefit contract, coverage document, or evidence of coverage) conflicts with the Policies, the Member’s contract shall govern. The Policies do not replace or amend the Member’s contract. Policy Limitation: Legal and Regulatory Mandates and Requirements The determinations of coverage for a particular procedure, drug, service or supply is subject to applicable legal and regulatory mandates and requirements. If there is a discrepancy between the Policies and legal mandates and regulatory requirements, the requirements of law and regulation shall govern. Reconstructive Surgery CA Health and Safety Code 1367.63 requires health care service plans to cover reconstructive surgery. “Reconstructive surgery” means surgery performed to correct or repair abnormal structures of the body caused by congenital defects, developmental abnormalities, trauma, infection, tumors, or disease to do either of the following: (1) To improve function or (2) To create a normal appearance, to the extent possible. Reconstructive surgery does not mean “cosmetic surgery," which is surgery performed to alter or reshape normal structures of the body in order to improve appearance. Requests for reconstructive surgery may be denied, if the proposed procedure offers only a minimal improvement in the appearance of the enrollee, in accordance with the standard of care as practiced by physicians specializing in reconstructive surgery. Reconstructive Surgery after Mastectomy California Health and Safety Code 1367.6 requires treatment for breast cancer to cover prosthetic devices or reconstructive surgery to restore and achieve symmetry for the patient incident to a mastectomy. Coverage for prosthetic devices and reconstructive surgery shall be subject to the co-payment, or deductible and coinsurance conditions, that are applicable to the mastectomy and all other terms and conditions applicable to other benefits. "Mastectomy" means the removal of all or part of the breast for medically necessary reasons, as determined by a licensed physician and surgeon. Policy Limitations: Medicare and Medicaid Policies specifically developed to assist Health Net in administering Medicare or Medicaid plan benefits and determining coverage for a particular procedure, drug, service or supply for Medicare or Medicaid members shall not be construed to apply to any other Health Net plans and members. The Policies shall not be interpreted to limit the benefits afforded Medicare and Medicaid members by law and regulation. Video Assisted Thoracoscopic Surgery (VATS) Sep 15 26