40Ar:r' diffusion in Fe-rich biotite Mlnrv Gnovn,c.ND T. M,c.Rx
advertisement

American Mineralogist, Volume 81, pages 940-951, 1996
40Ar:r'diffusion in Fe-rich biotite
Mlnrv Gnovn,c.ND
T. M,c.RxH,mnrsox
Department of Earth and SpaceSciencesand IGPP, University of California, Los Angeles,California 90024, U.S.A.
Arsrn^l,sr
Hydrothermal bulk-loss experiments employing radiogenic Ar 1oo4l*, were performed
to determine whether aoAr*diffusivity in biotite increaseswith Fe content. Diffusion laws
determined for intermediate and Fe-rich biotite assuming single-domain difrrsion (infinite-cylinder geometry) are remarkably similar: Fe-mica biotite (X*i*:0.71)
D:
0.401$.!$exp[-(50500+ 2.2)/RT] and Cooma biotite (X_",* : 0.54) D : 0.0751881?exp
[-(47100 + 1.5)/Rf]. The nearly identical resultsfor Fe-micabiotite and Cooma biotite
and their similarity to those from previous studies indicate that most biotite grains of
intermediate composition possesscomparable aoAr* diffusion properties. Becauselimited
grain breakageand volumetrically minor recrystallization is unavoidable during hydrothermal heating in bulk diffusion experiments, these diffusion laws necessarilyprovide
upper limits to 40Ar* loss by intercrystalline diffusion. The measured rates of aoAr* loss
from biotite agreereasonablywell with expectationsbasedon single-domain volume diffusion using infinite-cylinder geometry when experimental uncertainties are taken into
account. However, lack of information regarding aoAr* gradients within the hydrothermally treated mica prevents us from precluding more complex diffusion mechanismsinvolving high diffusivity pathways. In this paper we consider the significanceof bulk-loss
aoAr* diffusion experiments and discusshow diffusion parametersdetermined in the laboratory may be applied to thermochronology provided suitable constraints are available.
et al. 1967;Vedder and Wilkins 1969,Sanzet al. 1983).
40Ar* diffusion studies (Giletti 1974; NorThe ability to constrain temperature-time histories of Hydrothermal
rocks is of paramount importance for reconstruction of wood 1974;Harrisonet al. 1985;Hesset al. 1987;Onsthe evolution of the Earth's crust. Thermochronologic use tott et al. l99l) of biotite samplesof known composition
of 4oArl3eArdata for biotite or other phasesrequires ev- are indicated in Figure l In this paper we presentresults
aoAr* bulk-loss experiments peridence that radiogenic aoAr (ooAr*)loss in nature occurs from hydrothermal
formed with Fe-rich biotite (Fe-mica biotite; Govindarby a quantifiable diffusive processand availability ofapaoAr*diffusion studpropriate diffusion parameters(McDougall and Harrison adu 1979;Table l; Fig. l). Previous
(Norwood
ies
1974;
Harrison
1985)
et
al.
have predicted
1988).While field experimentsrelying upon the thermal
was
that
Fe-rich
biotite
significantly
less
retentive
of 40Ar'*
effectsof intrusions (e.g.,Westcott 1966;Hanson and Gast
than
intermediate
Fe'*/(Mg
*
Fe2+
compositions.
To
)
1967) unarguably representthe most direct approach for
aoAr* retentivity of Fe-rich biotite
understanding the rates and processesof o0Ar* loss in compare directly the
minerals under crustal conditions, difficulties inherent in and that of biotite of intermediate composition, addidetermining thermal historiesof crustal rocks rendersfield tional experiments using identical experimental techcalibration of diffusion laws problematic. Therefore, al- niques were performed on Cooma biotite; (Harrison et
though extrapolation of diffusion parameters measured al. 1985;Table l).
at higher temperaturesand shorter time scalesthan those
relevant to 40Ar*loss from minerals in nature is not withExprnrprnNTAl DESTcN
out risk (e.g., Villa and Puxeddu 1994), the large uncertainties associatedwith field calibration emphasize the
Diffusion coefficientswere calculatedfrom the bulk loss
need for laboratory studies.
of 40Art<from sized aggregatesof biotite hydrothermally
BecauseAr has extremely low solubility in silicate min- heatedat constanttemperature(e.g.,Giletti 1974).This
erals (Ozima and Podosek 1983), Ar diffusion experi- simple approach circumvents difficulties related to obments invariably employ natural aoAr* as the diffirsant. taining Ar measurementswith micrometer-scaleresoluHydrothermal experiments are required to study ooAr* tion and hasproducedresultscomparableto thoseof depth
retentivity in biotite becauseofbiotite's tendency to de- profiling (e.g.,Kelley et al. 1994a)in orthoclase(Foland
composewhen heatedin atmosphereor in vacuo (Brandt 1974). Fractional losses(/) were calculatedaccording to
0003-004x/96l0708-0940$05.00
940
INrnoougrroN
941
GROVE AND HARRISON: {AT* DIFFUSION IN FC-RICH BIOTITE
TABLE 1.
Biotite comPositions
Cooma- biotite
5l
r.rAl
16rAl
l2
_3
s
il
Fe3+
FeF+
os
00
Mn
Mg
K
Na
Total Cations
OH
02
04
06
08
Ft
ctt
l0
Mg(Mg+Fe)
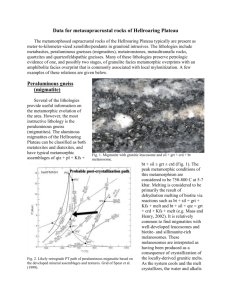
Frcunr l. Contour plot of compositions of biotite from a
wide spectrum of metamorphic rocks from subamphibolite to
granulite facies;plot is basedon data from Guidotti (1984). The
black area indicates the region ofthe plot in which >50/oofthe
analysesoccur. Note the predominanceof biotite of intermediate
composition. Labeled squaresrepresentcompositions of biotite
examinedin hydrothermul +0fu* studies:(1) Fe-mica biotite (this
study), (2) Cooma biotite (Harrison et al. 1985),(3) intermediate
biotite of Hess et al. (1987), (4) various biotite grains examined
by Norwood (1974), (5) Benson Mines biotite (Onstott et al.
l99l), and (6) F-rich phlogopite examined by Giletti (1974).
Note that new electron microprobe analysesobtained for the
biotite of Norwood (1974) are tabulated in Appendix Table l.
2.69
1.31
0.50
0.15
0.07
1. 1 9
0.02
0.98
0.94
0.02
7.87
1.77
0.12
0.11
Fe-mica-- biotite
2.72
1.28
0.53
0.15
0.27
1.27
0.02
0.54
0.91
0.04
7.73
1.59
0.40
0.01
where I and a represent the heating duration and measured radii of the grain, respectively. Neglecting additional terms causesan error of <0. l0 in natural log units
for values of Dt/a2 < 0.05. Activation energies(E") and
frequencyfactors (Do)were calculatedin the conventional
manner from the slope (-,8"/R) and ordinate (ln Do) of
an Arrhenius plot of the experimental results (ln D vs.
inverseabsolutelemPerature).
oF STARTTNGMATERTAT,S
CrHucrBmzATIoN
The compositions of both Fe-mica and Cooma biotite
were determined by wet-chemicalmethods and X-ray dif( l ) fraction (XRD) (Table l). Cell dimensions determined by
R.E. Jones(UCLA) usingXRD ate a : 5.335,b : 9.246,
c : 10.190A, P : 100.24 for Fe-mica,and a: 5.341,
b :9.237, c : 10.233A, P : 100.02"for Coomabiotite.
where u and h denote untreated and hydrothermally anSpot analysis by electron microprobe indicated homogenealed biotite, and "r is an experimentally determined
neousmajor elementcompositions.Both optical and backconstant characterizingproduction of3eAr from 3eKdurscatteredelectron imaging showed no detectablechlorite
ing neutron irradiation (McDougall and Harrison 1988).
sample (see also analysis performed by Tetley
o0Ar*
concentration within in either
Use of Equation 1 assumes
This is also supportedby high KrO contents(Ta1978).
aoAf
the grains is initially homogeneousand that external
ble l) and the absenceofbasal reflections from chlorite
concentrationsare maintained at zero (Crank 1975).The
in X-ray difraction patterns. These observations do not
latter is guaranteedby an infinite reservoir of atmospherpreclude the presenceof trace, nanometer-scalechlorite
36.4r
(a0Ar/36Arotr: 295.5)proic Ar. Measurementsof
(e.g.,Hess et al. 1987;Onstott et al. 1991).
o0Ar
incor- intergrowths
vided the basis for correcting for atmospheric
porated into the grains during hydrothermal treatment. Distribution of aAr*
Following earlierstudies(e.g.,Giletti 1974),diffusioncoCalculation of model diffusion coefficientsrequires hoefficients (D) were calculated with the assumption that
aoAr*in the host. Uniform aoAr*
aoAr* loss during hydrothermal treatment occurs by sin- mogeneouslydistributed
for Fe-mica becauseit is deexpected
are
concentrations
gle-domain, intracrystalline diffusion, with bulk o0Ar*
(Roubault et al. 1968)
intrusion
higlr-level
from
a
rived
transport normal to the c axis (infinite-cylinder geomehave cooled rapidly. Although the Cooma
to
is
likely
that
aoAr*
loss
try). The compatibility of the systematicsof
plutonic complex originated from somewhat deeper levwith these assumptions can be evaluated by comparing
els (-3 kbar; Vernon 1988),cooling at approximately20
the measuredvaluesof/with thosepredictedby the model
through its closure interval for Ar retention min"C/Ma
in plots of /vs. t/a2 or equivalent representations(Crank
potential for significant 40Ar*gradients(Tetley
the
imizes
1975). Diffusivities were calculated using the approxi1978). The 4o\r/3eAr step-heating experiments have
mate solution for the infinite cylinder of Reichenberg
yielded uniform age spectrafor both Cooma biotite (Te(l 953):
tley 1978) and Fe-mica (Harrison, unpublished data).
theseresults do not require the absenceof
(2) Unfortunately,
ooAr*
gradients
becausestructural decomposition
internal
:('-
(#,"J.^-(#),r
(HJ^
GROVE AND HARRISON' 4oAr* DIFFUSION IN Fe-RICH BIOTITE
942
Tlele 2. Cooma and Fe-micabiotitegrain-sizedimenstons
Meshsizet
Length
-L (001)
(pm)-.
100-120 318+ 65
120-140 294 + 71
140-170 247 + 59
60-80
80-100
100-120
120-140
140-170
170-200
200-230
488 +
398 +
313+
289 +
248 +
188+
143 +
99
84
72
82
73
45
53
width
r (001)
tum)-Cooma
202 +
166+
134+
Effective
radius
(pm)t
biotite
29
128 + 13
20
1 1 3+ 1 3
10
96 + 10
Fe-mica biotite
305 + 41
241 + 28
203 + 19
168 + 22
141+16
113r'12
88+11
Ptt2o= Prorut=1000 bars
-10
Length/width
1.6 + 0.4
1 . 8+ 0 . 5
1.8+ 0.5
An -15
00
191 + 23
154 + 17
125 + 14
109 + 15
92+19
72+9
56+10
1 . 6+
1 . 7+
1 . 6+
1 . 8+
1 . 8+
1 . 7I
1 . 6+
0.5
0.5
0.4
0.6
0.6
0.3
0.3
t Units are
division oer inch.
-t Results 100
of
measurementsper size fraction.
t EffeJtiveradii (a) calculatedfrom measuredflake dimensionsDy a :
\alwl2.
-20
-25
I Cooma Biotite
1Ann55)
r Fe-mica Biotite
(Ann 71)
-30
400
s00
600
700
E00
900
r("c)
Frcuns2. Z-/", stabilityrelationsof ferromagnesian
biotite.
Solid linesrepresentisoplethsfor annite + O, : magnetite+
of biotite by dehydroxylation and basal delamination
potassiumfeldspar+ HrO (valuesrepresentannitecontentof
during in vacuo heating obscuresaoAr* gradients by ho- biotite).Phaserelations
arecalculated
for HrO pressure
of 1000
mogenizingthem (Hansenet al. 1975;Gaberet al. 1988). barsusingdataofWonesand Eugster(1965).Buffersshownby
Both Fe-rich mica and Cooma biotite indicate homoge- the dotted lines are hematite+ magnetite(HM), Ni + NiO
neous aoArxconcentrationswhen sampled in bulk (-5
(I.{NO), qraftz + fayalite + magnetite(QFM), and gaphite +
mg) aggregates.Harrison et al. (1985) reported a 4oAr* methane(C-CH4).The solid squaresand circlesrepresentthe
conditionsfor CoomabiotiteandFe-mica,respecconcentrationof 6.001 + 0.009 x l0-e mol/g for three experimental
tively.
replicate analyses of Cooma biotite. Fe-mica (40Ar* :
4.384 x l0-e mol/g Govindaradu 1979)has been used
in the UCLA 40Ar/3eAr facility for over 6 yr as a concen- heated, cold-seal pressurevesselsunder conditions inditration standard for aoAr*. The 4oAr:*/3eArratios of ali- cated by previous experimental studiesto be within their
quots of both coarse- and fine-size fractions of Fe-mica respectiveP-T-fo, stability fields (Fig. 2; seeWones and
can be reproducedwith the sameprecision(=0.60lo).
Eugster1965; Rutherford 1973).Experimentswere conducted at 550-700 oC and 1000 bars (Table 3). In FeSizing
mica experiments, f and /o, conditions were maintained
Mica grains were sorted by dry and then wet sieving at values imposed by the graphite + methane buffer with
into sevensize fractions between 80 and 230 mesh. Most the use of thin-walled, AgroPdrocapsulesand methane
grains possessedsmooth basal cleavageswith essentially equilibrated with powdered graphite and graphite filler
vertical margins as measured from the basal cleavage. rods as the pressuremedium (Huebner 197l). In Cooma
Long and short dimensions of cleavagefaceswere mea- biotite experiments,sufficienttime (15-100 d) was likely
sured for at least I 00 grains from eachsizefraction (Table available to permit equilibration through the 0.25 mm
2). Diameter-to-flake thickness ratios estimated from thick gold capsulewalls with the intrinsic,fo, of the Stelknown KrO contents and 3eAryields obtained by fusing llte 25 pressure vessel and the stainless steel filler rod
measuredgrains with an Ar ion laser were typically 25: l, (approximately that of the Ni + NiO butrer; Huebner
in agreement with less precise optical determinations. l97l). Temperaturewas regulatedwith proportionalconGrain radii were estimated by calculating the area of the trollers using dual Chromel-Alumel thermocouples.Presellipse defined by the long and short dimensions of cleav- sure was monitored with a Heise-Bourdon gauge.After
age faces and then finding the radius of the equivalent equilibration, variations in these parameters were genarea cylinder (Harrison et al. 1985). An alternative ap- erally lessthan + I 'C and t5 bars. Temperature calibraproachusedby Giletti (1974)and Norwood (1974)yields tion performed using two thermocouplesagreedwith exa radius that is 12.80/o
larger and diffusion coefficientsthat pected values for the melting points of pure NaCl and Al
are 20o/osmaller (-0.24 natural log units on an Arrhenius wrre within 5 'C. All samples were quenched in complot). This discrepancyis comparableto the experimental pressedair and removed from the pressurevesselwithin
uncertainty in the measurement.
5-10 min of power shut off. Chargeswere discarded if
weighing routines indicated leakageof HrO.
HyonorrmnrvrAl, TREATMENT
Light microscope and SEM imaging of treated mica
Sized, 25 mg aliquots of biotite were sealedin either revealed both appreciable breakageof original grains as
gold or AgroPdrocapsuleswith I mg pure water. Isother- well as new biotite growth. In general, hydrothermally
mal, isobaric heating was performed within externally treated Fe-mica biotite tended to exhibit a higher per-
943
GROVE AND HARRISON' 4oAr* DIFFUSION IN Fe-RICH BIOTITE
TABLE3, Cooma and Fe-mica biotite experimentalresults
T
Expt.
fc)
(d)
lnD
(pm)
HCB-1
HCB-2
HCB.3
HCB-4
HCB-5
HC8-6
HCB-7
HCB-8
550
550
600
600
650
650
700
700
97.1
3
97.13
50.29
50.27
28.91
28.95
15.89
15.93
Cooma biotite
96+10
1 1 3+ 1 3
1 1 3+ 1 3
128+ 13
1 2 8l 1 3
129 + 13
128+ 13
1 2 8+ 1 3
0.102+
0.074+
0.159+
0 . 1 1 1+
0.181+
0.180+
0.241+
0.233+
0.006
0.004
0.004
0.004
0.005
0.004
0.003
0.006
-31.88+
-31.70 +
-29.49 r
-29.98 +
-28.42 +
-28.43 +
-27.22+
-27.30 !
Fm-12
Fm-1
Fm-9
Fm-3
Fm-10
Fm-2
Fm-5
Fm-4
Fm-6
Fm-8
Fm-15
Fm-11
Fm-13
Fm-14
Fm-16
Fm-17
700
700
700
700
700
650
650
650
650
600
600
550
550
550
550
550
78.09
15.07
22.01
15.07
14.03
25.88
44.16
20.98
14.86
23.91
75.93
78.09
75.93
75.93
67.92
67.92
Fe-mica biotite
154+ 17
129 + 14
109+ 15
92+13
92+13
125 + 14
125 ! 14
92+13
92+10
56r10
56+10
109+ 15
92+13
56+10
56110
56+10
0.584+
0.307+
0.250+
0.349+
0.302+
0.191+
0.216+
0.185+
0.121r
0.216+
0.290+
0.0443+
0.0948+
0.117+
0.106+
0.135+
0.003
0.006
0.006
0.006
0.006
0.006
0.006
0.004
0.007
0.007
0.007
0.0101
0.0076
0.009
0.007
0.007
-26.49 + 0.22
-26.70 ! 0.23
-27.79 + 0.29
-27.O4+ O.29
-27.28 + 0.29
-28.25 ! 0.24
-28.52 + 0.24
-28.71 + 0.30
-29.24 + 0.32
-29.51 + 0.38
-30.04 a 0.38
-32.60 + 0.60
-31.38+ 0.34
-31.93 + 0.42
-32.03 + 0.40
-31.54+ 0.39
centageof new gain growth. XRD patterns obtained for
all experiments revealed only biotite peaks. Ultrasonic
treatment and wet sieving under acetonewas performed
to recover from the capsulesgrains with dimensions similar to those of the original starting material. Only the
mica grains with dimensions corresponding to those of
the original size fraction (approximately 60-900/oof the
treated grains) were used in further analysis.SEM imaging of thesematerialsindicatedthat <5-100/oof the surface of treated grains was covered by neoformed, l-10
pm diameter hexagonal platelets after ultrasonic treatment (Fig. 3). Note that original broken grain boundaries
are well preserved even after heating at 700'C for 78 d
(Fig. 3a). Close inspectionrevealsthat micrometer-scale
biotite overgrowths forming euhedral margins are common on broken grain edges(Fig. 3b). Recrystallization on
this scale probably represents < lolo of the mass of the
original grain. For example, reprecipitation of biotite to
cover 100/oof the surface with 10 pm diameter (l pm
thick) grains correspondsto 0.400/orecrystallization.
0.25
0.32
0.25
0.24
0.22
O.22
o.21
0.2'l
GP-50 Zr-Al getter pump and analyzedwith a Nuclide
4.5-60-RSS mass spectrometer operated in the Faraday
mode [aoArsensitivityof 1.5 x 10-15mol/mV; massdiscrimination of 0.994 per amu; seeHarrison and Fitz Gerald (1986) and McDougall and Harrison (1988) for additional experimental details]. In each analysis, samples
were degassedin a crucible preheatedto 350'C for -5
min to melt the enclosing tin foil' Although this gas was
generally not analyzed, measurementsperformed for the
first severalsamplesdetectedbackground3eArvalues and
atmospheric 40Ar/36Arratios. After pretreatment at 350
'C, temperaturewas increasedto 850'C for -5 min to
stabilize the mica grains by allowing them to dehydroxylate (Vedder and Wilkins 1969) before fusing them for
-5 min at 1350'C. Although, blank determinationsfollowing this procedurewere < l0 x l0-'a mol at m/e 40,
<2 x l0-t6 mol at m/e 39, and < I x l0-'u mol at m/e
36, sporadically low-percent aoAr*yields measuredfor all
materials indicate somewhat higher degrees of atmospheric contamination.
&Ar/3eAl ANALYSTS
RBsur,rs
Hydrothermally treated Fe-mica and Cooma biotite
were analyzed for o0Ar* loss using the 40Ar/3eArtechnique. Hydrothermally treated mica grains interspersed
with untreated biotite and Fish Canyon sanidine (FCT1) were irradiated for 120 h in the L67 position of the
Ford reactor (University of Michigan). These conditions
yielded "I factors of -0.02 (Table 4) and necessitateda
correction factor for K-derived o0Arof 0.025 (determined
from measurementson KrSOo). Gas extracted from -5
mg splits of mica wrapped in tin foil in a double-vacuum
tantalum resistancefurnace was purified with an S.A.E.S.
Results of the ooArl'nAr analysesof untreated and hydrothermally annealed Fe-mica and Cooma biotite are
presented in Table 4. Note that approximately 300/oof
the analyseswere performed on untreated mica. Total
fusion ageswere calculated with ,I factors determined by
assigning an age of 27.8 Ma to Fish Canyon sanidine
(Cebulaet al. 1986).The ageuncertaintiesinclude a 0.50/o
analytical uncertainty in "L Weighted mean agesand standard errors calculatedfrom separatealiquots ofuntreated
biotite were 307.6 + 0.4 Ma (eight analyses)and 398.1
+ 0.7 Ma (19 analyses)for Fe-mica biotite and Cooma
944
GROVE AND HARRISON' eAr* DIFFUSION IN Fe-RICH BIOTITE
Frcunr 3. Representative
SEM imagesof hydrothermally
heatedbiotite. (a) Grain from experimentFm-12, which was
heatedat 700'C for 78 d (longestdurationat 700.C).Notethat
original fracturedgrain-boundarymorphologiesare well preserved.Note that the cleavagesurfaceis affectedby new mrca
grofih of micrometerscale.As discussed
in the text, recrystallization on this scaleprobablyrepresents<10/oof the massof
the originalgrain.(b) Closeinspectionofmany brokensurfaces
revealsneweuhedral
biotiteovergroMhsof submicrometer
scale.
Notethatan -0.5 pm thick overgrowthcoveringtheentiregrain
wouldcorrespondto only 1olo
recrystallization.
biotite, respectively.Theseresultscompare favorably with
previousK-Ar agedeterminationsof 307.3 + 2.0 Ma for
Fe-mica biotite (Harrison, unpublished data) and 398.8
+ 2.5 Ma for Cooma biotite (Tetley 1978).The homogeneouscharacter of the starting material is indicated by
<0.30/oreproducibility in the total gas ages yielded by
individual analyses(Table 4). The averageuncertainty in
total fusion age (excluding analytical uncertainty in,f is
also 0.30/0.Duplicate analyseswere generally performed
for treated mica from each experiment. Although only
40o/oof the total fusion ages calculated from duplicate
analysesagreed to within 0.30/0,nearly 800/oreproduced
to within l0l0. Total fusion ages from hydrothermally
treated Fe-mica biotite were more difficult to reproduce
than those from treated Cooma biotite (Table 4).
Fractional loss (/) values for the hydrothermally treated mica calculated with Equation I are shown in Table
3. Most values of / were computed from the averageof
two replicate analysesfrom the same experiment. Propagation of errors associatedwith40Ar*/3eAr and,Iindicate
that relative uncertainties in fractional loss values in the
range0. l0 < /< 0.50 are -5o/o(on the order of +0.01
units in fractional loss). The uncertainties in fractional
loss for a given experiment appear to account for overall
experimental reproducibility. For example, values of
fractional loss determined for two setsof nearly identical
experiments(HCB-5 and HCB-6, HCB-7 and HCB-8)
agreedto within 0.4 and 3.50/0,respectively(Table 3). For
fractional loss values <0.10, relative uncertaintiesapproach 25o/o.
Model diffusion coemcients(D) calculated with Equation 2 are also shown in Table 3. Uncertainties reported
for D are typically +0.3 natural log units and reflect + lo
uncertainties in both fractional loss and estimated grain
size (Table 3). Note that asymmetric uncertainties in ln
D were rounded in Table 3. At a given temperature, experimental reproducibility (+0.S natural log units) is
nearly accounted for by experimental uncertainty at the
+2o level. This scatter likely results from minor (< lolo)
recrystallization during hydrothermal treatment and from
the presenceof small grain fragments formed during initial experimental pressurization but not removed during
subsequentultrasonic cleaning and resizing.
Least-squaresregressionofthe Arrhenius data for Femica using a + 5 'C uncertainty in temperature yields
50.5 + 2.2 kcal/mol and 0.40191fcm,/s (MSWD : 2.1)
for E" and Do, respectively (Fig. 4a). Following the same
procedure for Cooma biotite yields an E^ of 44.5 + 2.5
kcal/mol anda Do of 0.01519$fr!
cm,/s (MSWD:0.57).
These values are within 1o of those obtained by Harrison
et al. (1985), who reported 47 + 2.1 kcal/mol and
0.0771834cm2ls for E^ and Do, respectively.Combining
the data obtained for Cooma biotite in the two studies
yieldsvaluesof 47.1+ 1.5kcal/moland0.0751ggjf
cmrls
(MSWD : 1.25)for.E" and Do, respectively.Considering
the large experimental uncertainty, these values are essentially the same as those obtained for Fe-mica biotite.
The higher degree of scatter exhibited by Fe-mica may
reflect greaterrecrystallizationand comminution of grains
than was observedin experimentsperformed with Cooma
biotite.
Drscussrolt
Significance of biotite bulk-loss {Ar* diffusion
experiments
Taking experimental uncertainty into account, loss of
aoAr* from biotite in the present experiments agrees
reasonably well with expectations based on the singledomain diffusion model with infinite-cylinder geometry.
This compatibility is illustrated in Figure 5. In this diagram, values of t/a2 calculated from the measuredgrain
size and heating duration are plotted against measured
fractional loss. Also shown are single-domain solutions
for the infinite cylinder at the indicated temperatures.As
GROVE AND HARRISON: aoAr* DIFFUSION IN Fe-RICH BIOTITE
945
TABLE4. Cooma and Fe-mica biotite 10Ar/3eAr
analytical results
Expt.
Jtactor
Weight
(ms)
HCB-1
HCB-2
HCB.2
HCB-3
HCB-3
HCB.4
HCB.4
HCB.5
HC8-6
HC8-6
HCB.7
HCB-7
HCB-8
HCB-8
Untreated
Untreated
Untreated
Untreated
Untreated
Untreated
Untreated
Untreated
0.02054
0.02055
0.02055
0.02055
0.02055
0.02055
0.02055
0.02055
0.02057
0.02057
0.02057
0.02057
0.02058
0.02058
0.02054
0.02054
0.02054
0.02056
0.02056
0.p2060
0.02060
0.02060
3.45
3.40
5.87
5.49
6.83
5.47
3.66
2.91
5.48
5.43
9.93
5.64
5.45
7.16
3.87
6.07
6.49
6.01
6.98
6.25
10.10
5.51
Fm-1
Fm-1
Fm-2
Fm-2
Fm-3
Fm-3
Fm-4
Fm-4
Fm-5
Fm-5
Fm-6
Fm-6
Fm-8
Fm-9
Fm-9
Fm-10
Fm-10
Fm-11
Fm-11
Fm-12
Fm-12
Fm-13
Fm-l3
Fm-14
Fm-14
Fm-15
Fm-15
Fm-16
Fm-16
Fm-174
Fm-17A
Untreated
Untreated
Untreated
Untreated
Untreated
Untreated
Untreated
Untreated
Untreated
Untreated
Untreated
Untreated
Untreated
Untreated
Untreated
Untreated
Untreated
Untreated
Untreated
0.02087
0.02087
0.02094
0.02094
0.02103
0.02103
0.02107
o.02107
0.02044
0.02044
0.02042
0.02042
0.02040
0.02039
0.02039
0.02038
0.02038
0.02037
0.02037
0.02037
0.02037
0.02037
0.02037
0.02038
0.02038
0.02040
0.02040
0.02041
0.02041
0.02042
o.02042
0.02046
0.02046
0.02046
0.02038
0.02038
0.02039
0.02039
0.02039
0.02044
0.02044
0.02044
0.02046
0.02046
0.02082
0.02082
o.02082
0.02114
0.02114
0.02114
3.77
7.37
3.19
6.07
4.36
4.81
4.00
3.56
sAr x 10-12mol
Equivalent
V" K2O
Cooma biotite
10.3
1.89
10.0
1.81
9.2
2.90
9.7
2.84'
o',
3.54
10.2
2.99
10.7
2.09
10.1
1.57
9.9
2.89
2.83
9.7
9.5
5.05
9.8
2.95
1
0.0
2.91
o'l
3.70
9.8
2.02
v.o
3.11
9.8
3.41
9.6
3.10
9.5
3.56
9.4
3.16
9.1
4.91
10.2
3.01
o/o QAf'
74.5
Jb.5
81.6
68.7
76.6
85.6
78.9
55.7
65.3
76.6
83.6
77.6
67.8
76.1
91.3
86.4
91.8
92.0
84.8
74.3
86.8
85.8
{Ar'fsAr
10.79
11.17
11.07
10.12
10.09
10.71
10.65
9.84
9.84
9.84
9.11
9.11
9.17
9.24
12.01
12.06
12.02
12.00
11.98
12.00
11i96
12.00,
Apparent
age(Ma)
361.1+
372.8!
369.8+
340.9+
339.9+
358.9+
357.1+
392.2+
332.5l
332.5+
309.9+
309.9+
3 1 1 . 9+
3 1 4 . 1+
397.8+
399.3r
398.1+
397.8+
397.2+
398.5+
397.3+
398.5+
2.2
2.1
1.8
1.7
1.7
1.7
1.7
1.6
2.0
1.6
1.6
1.7
1.7
1.6
1.9
1.9
1.9
1.9
1.8
1.8
2.0
2.0
Fe-mica biotite
J.5Z
5.47
4.48
7.31
5.26
4.52
4.36
6.16
J.CO
4.59
/.oc
6.69
5.85
1.89
4.37
3.14
1.26
2.05
3.70
6.29
4.06
5.00
5.38
5.85
4.30
2.83
8.09
2.65
5.40
3.24
5.80
4.3
3.85
2.62
5.28
7.20
+.c I
5.89
2.45
7.13
5.85
7.45
2.00
3.52
1.71
3.11
1.14
2.33
2.04
1.32
2.69
2.74
2.25
3.43
2.46
2.30
2.24
2.78
1.69
2.10
3.27
3.52
3.25
0.891
2.18
1.59
0.634
0.664
1.68
3.01
2.07
2.49
2.61
2.97
2.10
't.21
4.27
'l.29
2.68
1.58
2.99
2.12
1.90
1.35
2.40
3.55
1.97
2.88
1.30
3.61
2.91
3.41
9.8
8.8
9.8
9.4
4.8
8.9
9.3
6.8
9.5
9.4
9.5
8.8
8.8
9.6
9.7
8.5
9.0
8.6
8.1
9.9
10.5
8.9
9.4
9.6
9.5
6.1
8.6
9.0
9.6
9.4
9.1
9.5
9.2
8.0
10.0
9.2
9.4
9.2
9.7
9.3
9.3
o7
8.5
9.3
8.0
9.0
9.8
9.2
9.1
8.3
50.9
50.3
73.5
73.4
68.1
63.9
84.8
61.2
69.1
82.0
81.6
84.4
oc.c
85.0
74.9
88.0
63.3
39.4
42.9
72.4
64.9
86.3
84.8
77.1
41.9
79.8
70.8
77.8
77.2
69.7
79.1
96.6
88.9
67.4
94.0
93.5
83.4
63.3
68.2
78.0
85.1
77.8
62.7
90.8
84.4
89.7
29.5
86.2
95.2
72.5
6.18
6.16
7.18
7.19
5.70
5.81
7.20
7.18
7.14
7.12
8.00
8.00
7.14
6.80
6.87
6.35
6.38
8.70 ',
8.74
3.82
3.78
8.32
8.20
7.88
8.22
6.57
6.36
8.11
8.17
7.81
7.94
9.18
9.08
9.07
9.11
9.23
9.09
9.13
9.09
9.11
9.09
9.07
9.08
9.12
8.91
8.93
8.63
8.75
8.80
8.80
218.9I 1.4
218.2! 1.2
252.7+ 1.3
253.0+ 1.2
204.2+ 1.2
208.0+ 1.2
254.8+ 1.4
254.2! 1.5
245.8!',t.2
245.1+ 1.2
273.0! 1.4
273.0+ 1.4
245.3+ 1.3
2U.3 + 1.1
236.5J 1.5
2 1 9 . 6+ 1 . 1
2205 + 1.2
294.3+ 1.6
295.6+ 2.3
135.2+ 0.7
133.8+ 0.7
282.4! 1.6
278.7! 1.4
'268.7+ 1.4
279.4+ 2.3
226.9! 1.4
220j + 1.4
276.3+ 1.3
278.2+ 1.4
267.O
! 1.4
271.'l! 1.4
319.5+ 1.6
307.4+ 1.5
307j + 1.7
307.2+ 1.5
3 1 1 . 0+ 1 . 7
306.8+ 1.7
308.0I 1.5
306.8+ 1.5
308.1l 1.9
307.4+ 1.5
306.8+ 1.5
3O7.4+ 1.7
308.7r 1.5
307.0+ 1.7
397.6+ 1.5
298.1+ 2.6
306.2l 1.5
307.8+ 1.6
397.9+ 1.5
946
GROVE AND HARRISON'4Ar*
DIFFUSION IN Fe-RICH BIOTITE
(a) Fe-mica Biotite
-25
{b) Cooma Biotite
f
Opensymbolsfrom 6
Harrisonetal.(1985)O
+
- La t1
rJ
\vr\ I
d
\
\T
Y
\U)
c\
((),-
a
5 -29
202 pm
125gm
A
113pm
V
97pm
V
96Pn
o
o
sopm
56um
tr
-31
E, = 50.51 Z.ZkcdJmoI
E^= 47.I + 1.5kcaVmol
l n D o = - 6 . 9 *11 . 2
lnDo=-2.59*6.3a
MSWD=21
MSWD= 1.3
-JJ
-JJ
0.95
r.00 1.05 1.10 1.1_51.20 1.25
1000/r(K)
0.95 1.00 1.05 1.10 1.15 t.20 1.25
1000/r(K)
Frcunp 4. Arrhenius plots for Cooma biotite and Fe-mica
(infinite-cylinder model). (a) Diffusion coefrcients calculatedfor
Fe-mica. (b) Resultsfor Cooma biotite, including data from Harrison et al. (1985). Note that the values are nearly identical to
those for Fe-mica biotite. Results obtained for Cooma biotite
(solid symbols) in the presentstudy agreewith those of Harrison
et al. (1985; open symbols)\ /ithin the experimental uncertainty,
except for the a : 202 pm size fraction. Analytical uncertainties
are lo and reflect uncertainties in fractional loss and calculated
grain radii. The reproducibility ofdiffusion coefficientsat a given
temperature is explained by experimental uncertainty at the 2o
level. Much of the variability is believed to result from minor
recrystallizationand the presenceofgrain fragmentsformed during initial pressurizationthat remained after ultrasonic cleaning
and resizing.
indicated in Figure 5, the majority of the results agree
with the infinite-cylinder solution within experimental
uncertainty.
An alternative interpretation, advanced by Villa and
Puxeddu (1994), of the mechanismsgoverning a0Ar*loss
from biotite in hydrothermal experimentsholds,6u1 +0fu*
is liberated primarily as a result of dissolution-reprecipitation reactions.These workers note that the dissolution
rates (ftu,..)indicated by Wood and Walther's (1983)Arrhenius-typerelationship[3.5 x l0 t2to 1.2 x l0-" mol
biotite(cm'z.s)over the temperaturerange 550-700 'Cl
predict substantial to complete dissolution of the biotite
in hydrothermal experiments performed under conditions similar to those employed in the presentstudy. The
low solubility of biotite at 500-700 "C, 1000 bar conditions (Frantz et al. l98l) and the 40loby weight HrO content ofthe chargesrequire that nearly all dissolvedbiotite
be precipitated as new grain growth. Preservationoforiginal grain morphologies and lack of evidence for volumetrically significant new grain gpowth in the presentexperiments indicate that values of ko,""obtained from the
Wood and Walther (1983) model are significantlyoverestimated for biotite under these conditions. For exam-
ple, the minor textural changesnoted for Fm- l2 (Fig. 3),
which was heatedat 700'C for 78 d, are grosslyinconsistent with the 580/orecrystallization that would be required to explain Ar loss by this mechanism. Moreover,
the activation energy determined for 40Ar* retention in
biotite grainsexaminedin this study (47-51 kcal/mol) is
significantlyhigherthan the 13.3kcal/mol activation enthalpy requiredby the Wood and Walther (1983)model.
The apparent inability of the dissolution model to account for the experimental results may be explained by
difficulties inherent in estimating effective surfacearea of
grainsand failure to adequatelyaccountfor deviation from
steady-stateconditions causedby damagedgrain surfaces
(Helgesonet al. 1984;Kerrick et al. 1991).Valuesof ku,.,
for steady-statesilicate hydrolysis,for example,have been
found to difer by more than four orders of magnitude at
constanttemperature(Murphy and Helgeson1989).
Incongruent dissolution of K representsan additional
potential pitfall. Becausefractional loss is calculatedfrom
changesin the a0Ar*/3eArratio rather than from measured
differencesin 40Ar* concentrations,values of /are erroneous if the K content of mica is altered by incongruent
dissolution during hydrothermal treatment (Hess et al.
GROVE AND HARRISON: aoAr*DIFFUSION IN Fe-RICH BIOTITE
947
(a) Fe-micaBiotite
0.5
0.5
a
CA
750"C
I
700"C |
7500C
650 0C
O
I
rcO"C
-+
^. 6000c /
Y sso"c/
r'so"c
f
8 0.4
0.4
J
"l
E
0.3
I
I
tr
L
-
o
r?,
t).2
{)?
-+
0.0
1o1o
lo11
t0t2
t/a2 (slcmz)
550
(l ?
0.1
0.1
600 0c
-c
0C
+
-e
0.0
10e
'+
-+
Open symbols fiom
Harrisonct al. (1985)
lolo
loll
1012
t/a2 (slcr#)
Fretnp 5. Plots offractional loss (f) vs. t/a2, where a and I
represent the measured grain radius and heating duration, respectively. Error bars reflect propagation of uncerlainties associated with a and the experimentaluncertainty in I Solid curves
are calculated for the single-domain, infinite-cylinder model at
the indicated temperatures.Results for Fe-mica biotite are indicated in a and those for Cooma biotite are shown in b. Open
symbols representresults of Harrison et al. (1985). Open symbols with dots represent the a : 0.0202 cm size fraction. Note
that experimental results for both Fe-mica and Cooma biotite
define nearly two orders of magnitude variation in t/a2 arrdgenerally fall within lo of the solution of the infinite-cylinder model.
This relationship reveals compatibility of the results with the
single-domain diffusion model.
1987). Although imprecise (+100/o),equivalent percent
KrO can be calculated from the 3eAr yields of both untreated and hydrothermally heatedbiotite (Table 4). Values obtained for untreated and treated Fe-mica biotite
(9.t + 0.6 and 9.1 + 0.9 o/oKrO,respectively)are identical and similar to the 8.8 t 0.4 o/oKrOvalue reported
by Govindaradu (1979). Similarly, percent KrO values
obtainedfor Cooma biotite (9.6 + 0.3 and 9.9 + 0.4 for
untreated and treated grains, respectively)agreewell with
the 9.8 o/oKrOvalue reported by Tetley (1978). These
results indicate that incongruent dissolution of K can be
ruled out at the l0o/olevel.
Uncertainty regarding the nature of 4oArrtconcentration gradients developed in the hydrothermally treated
mica prevents alternative diffusion mechanismsfrom being precluded. Use of natural biotite presents special
problems in that both the diffusion geometry and the actual length scalesof diffusion are not known with certainty. Moreover, extended defects and intergrown chlorite
or similar phasesin biotite may also significantly influence these parameters.Interestingly, laser-spot-profiling
studies of naturally heated, millimeter-scale biotite and
phlogopite(Phillipsand Onstott 1988;Onstottet al. 199l;
Phillips l99l;Kelley et al. 1994b)and other micas(e.g.,
Hodges et al. 1994) have reported aoAr* loss profiles that
appear inconsistent with those predicted for single-domain volume diffusion. Although some intragrain discontinuities are typically imaged, o0Ar* concentration
gradients are predominately developed normal to grain
boundaries over length scalestypically correspondingto
grain dimensions. Additionally, integrated ages tend to
be somewhat younger than those predicted by single-domain intracrystalline diffusion. These observations have
been cited as evidencefor the existenceofhigh-diffusivity
pathways operating over length scalescorresponding to
grain dimensions(Onstott et al. l99l; Lee 1995).Unfortunately, similar detailed laser-spot-profiling of hydrothermally treated mica grains examined in the present
study to determine concentration gradients was precluded by their small dimensions.Moreover, use of coarser
materials is limited practically by the minimal amount
of bulk aoAr* loss (- lolo)that can be induced during hydrothermal heating(=200'C) on laboratory time scales
(e.g.,Onstottet al. l99l).
An apparent discontinuous relationship between grain
dimensions and apparent aoArretentivity not anticipated
GROVE AND HARRISON' 40tu,| DIFFUSION IN Fe-RICH BIOTITE
948
Lb
Cooma
t4
a
-
t2
Biotite
(650'C
Tlau 5. Calculation of effective radii of diffusion
data)
a (cm)
MssnedD,
Dla'z(1ls)
Db(cm,is)t
a. (cm)'.
0.0202 1.0000
Erpeilmentalf
2 . 6 4 7x 1 0 e
1.08x 10-12
0.0147
0.0150 0.4095
0.014s 0.5905
butkl Dl*
Distribution 1
2.539 x 10-o
2.722 x 10-e
2 . 6 4 7x 1 0 e
1.08 x 10-i2
0.0147
0.0201 0.9852
0.0026 0.0148
butkl Dl*
Dist.ibution 2
1 . 4 1 4x 1 0 - e
8.483 x lo-t
2.647 x 1g-e
1.08 x 10-'e
0.0147
O
MDD Model
MP Mdel
I
4.08
SinSle-Domain
Diffusion
Model
(4=007?cm'zA)
Single.Dodn
Ditrusion Model
(4=0rxoc#rO
00
t,
50
r50
100
200
?so
a\w)
Frcunr 6. Measuredor bulk diffusioncoemcients(Do)of
Coomabiotite in 650 .c experiments
of the presentstudyand
Harrisonet al. (1985)plottedasa functionofgrain size(a).The
horizontallines representtwo solutionsfor single-domain
diffusionusingan activationenergy(,E-")
of 47 kcal/molandpreexponentialfrequencyfactors(Do)of 0.077and 0.040cm2ls,respectively.The dashedline representsa solution from the
multipath(MP) modelcalculated
by l-ee[1995;seeTable2 and
Fig. 4 ofLee (1995)for detailsl.The solidline represents
a solution from the multiplediffusiondomain(MDD) modelwith a
domaindistributionconsistingof a singlelargedomainaccounting for 98.50/o
of the volumeand smaller,a: 0.0015cm domainstotaling1.5volo/o.In all instancesthe dimensionsof the
largestdomainin the MDD modelare negligiblysmallerthan
actualgraindimensions.Both solutionsprovidean equallysatisfactoryfit to the observeddata.The MDD solutionindicates
a preexponential
frequencyfactor(Do)of -0.040 cm2lsandimpliesthat the valueofDo determinedfrom regression
ofexperimentaldatafrom all temperatures
(0.077cm2ls)wasoverestimatedby a factorof two.
by single-domain intracrystalline diffusion models has
been observed in hydrothermal experiments. For example, experiments by both Giletti (1974) and Harrison et
al. (1985)indicatedthat Arrhenius resultsfrom relatively
coarse-grainedmica (a > 200 rrm) were found to plot at
systematically higher diffusivities than those calculated
for finer grained mica (Fig. a). Note that only grains < 150
pm were usedin the presentstudy.Harrison et al. (1985)
interpreted the enhanced diffusivity exhibited by the
coarse mica to indicate the existenceof an effective diffusion radius (c.n) of - 150 pm. Similar discontinuous
relationshipsbetweengrain dimensionsand apparent40Ar
retentivity have also been observedin total fusion studies
of biotite heatedin nature (e.g.,Wright et al. l99l).
Transport of 40Art<along high-diffusivity pathways operating over length scalescorrespondingto grain dimensions during hydrothermal heating has been argued for
by Onstott et al. (1991)and others.Lee (1995)presented
calculations that demonstrate that increase in bulk diffusivity with grain radius can be explained as being a
direct manifestation of high-diffusivity pathways in the
multipath model (MP; Lee and Adama 1992).In the MP
model, progressivelymore 40Ar* is able to diffuse out of
the crystal along interconnected, high-diffusivity path-
Notej Calculations are based on 4 : 47000 calimol and Do: 0.077
cm'?/s.A volume diffusivity ol 5.71 x 10-13cmr/s was determined from
the Arrheniusequation[D: 4 exp(-ElRI)] at 650 rc. Volumefractions
cafculatedassuming cylindricalshape with a:l : 10:1, where / is one-half
height of the cylinder. Individualdomains sum to volume ot a : 0.0202
c m c y l i n d e (r a : / : 1 0 : 1 ) .
. Bulk difiusivitycalculatedfrom bulk (D/d) value using a = 0.0202 cm.
.'Effective radius(ad) for Ndiffusion domainscalculatedwith l/acd:
28,f,|4.
f Results for experiment CB-o in Table 2 of Harrisonet al. (1985).
I Bulk Dld calculatedfor /Vdifiusion domains calculatedwith D/#o :
2l!1 fDla|.
ways as the overall grain dimensions increase (Fig. 6).
We emphasizehowever, that the observed behavior can
also be easily explained with the multiple diffusion domain model (MDD; Lovera et al. 1989; Lovera 1992).
An argument typically employed againstthe possible existence of an effective diffusion radius for biotite is the
failure to recognize compositional subdomains in 40Ar/
3eArlaser-spotfusion studies(e.g.,Phillips and Onstott
1988).However, the conceptofan effectivediffusion radius necessarilyrequires multiple diffusion domains that
differ in dimension. In the MDD model, many domain
distributions are capable of explaining the anomalously
high diffusivities exhibited by the coarse-sizefractions.
These include distributions in which the largestdomains
are comparable in dimension to the observed grain size
(Table 5).
To demonstrate, consider the specific example employed by Lee (1995; resultsof Harrison et al. 1985 reproduced in Table 5). In this experiment, the diffusivity
calculatedfrom the experimentalresultsusingc :0.0202
cm was higher by a factor of about two than the values
yielded by smaller grains. The value of a"u required to
reconcilethe resultsin this instanceis 0.0147 cm. Note
that although the results can be modeled with a distribution of length scalescomparable to a"n(distribution I
in Table 5), an equally plausible distribution consisting
of a single large domain and a significantly smaller one
also provides a satisfactoryfit (distribution 2 in Table 5).
In fact, many such distributions exist in which small domains not resolvable by aoArl3eArlaser-spot-profiling
techniques increase bulk diffusivity. Given that typical
spot sizesemployed during 40Ar/3eArlaser-profiling studies are -25 trm, a grain characterizedby such a distribution would appear to have a0Ar* loss gradients controlled by grain boundaries and would yield a total gas
GROVE AND HARRISON: {AT* DIFFUSION IN FE-RICH BIOTITE
agelower than that anticipated from a single-domaindiffusion model.
In the bulk-loss experiments,the Arrhenius parameters
were determined following the assumption that eetrcorrespondsto measuredgrain dimensions (a-.".). The value
obtained for Do by regressingthe experimentally determined bulk diffusivities representsan upper limit to the
extent that a"o is smaller than the measured than 4-"",.
This relationship is illustrated in Figure 6. Here, grains
from each size fraction were consideredto be characterized by a single large domain correspondingto 98.50/oof
the total volume of the grain and a smaller (a : 0.0015
pm) domain accountingfor the remaining 1.50/0.
With Do
equalto 0.040 cm,/s insteadof 0.077 cm2ls,the fit to the
experimental data is equally satisfactoryas that provided
by the multipath model, implying that the value for Do
was overestimatedby a factor of two. Note that although
the existence of effective radii of diffusion is supported
by apparent age-grain size relationships observedfor biotite heatedin nature (Wright et al. l99l), the existence
of the smaller domains in the experimentally heatedmica
could represent an experimental artifact: Incomplete removal of broken grains formed as the experiments were
initially pressurized.
949
Values of c"n can be estimated for a biotite of interest
by comparing its difusivity determined in a hydrothermal experiment with that calculated from appropriate
Arrhenius parameters (Table 5). For example, Copeland
et al. (1987)calculateda"n: 0.034 cm for an intermediate
biotite [Fe,.,/(Fe,.,+ Mg) : 0.49, a : 0.08 + 0.02 cm]
from the Quxu pluton, southern Tibet, from the 7.80/o
aoAr* loss incurred in a single hydrothermal experiment
(T : 710 oC, A1 : 5.15 x 105s, / : 0.078) and by
assuming applicability of Arrhenius parameters from
Harrison et al. (1985).Assuming l0'C/Ma cooling,this
correspondsto a closure temperature of 335 'C. Villa and
Puxeddu (1994) performed similar experiments(T : 7 l0
" C , A t : 5 . 1 1 x 1 0 5s ) w i t h a - 0 . 0 0 6 5c m g r a i n so f
+ Mg) : 0.50] biotite from the
intermediate [Fe,o,/(Fe,o,
Larderello geothermal field. Although values of a.o calculated from these results agreed favorably with measured grain dimensions,Villa and Puxeddu(1994) concluded that aoAr* loss incurred during hydrothermal
heating resulted from dissolution processesand that bulk
closure temperatures for biotite in nature were significantly higher than those indicated by experimentally determined diffusion parameters.
As discussedbelow, it appearsthat most intermediate
biotite compositions (Fig. l) have similar Arrhenius paApplication to thermochronology
rameters. However, large uncertainties regarding approSingle-domain, intracrystalline diffusion representsthe priate Arrhenius parameters for nontypical biotite comsimplest model available for Ar transport in biotite or positions indicate that caution should be applied in the
other silicates that possessthe predictive value required estimation of values of a.nfor thesematerials in the manfor thermochronology. Calculation of bulk closure tem- ner describedabove. For example,Onstott et al. (1991)
peratures from experimentally determined diffusion pa- employed different extrapolationsbetweenGiletti's ( I 974)
rameters is straightforward when a single-domain diffu- Arrhenius parametersfor phlogopite and those for intersion model is employed(e.g.,Goodwin and Renne 1991; mediate biotite compositions(Norwood 1974; Harrison
Hesset al. 1993).For example,bulk closuretemperatures et al. 1985) for Benson Mines phlogopite [Fe,.,/(Fe,.,+
of 310 t 40 and 330 + 50 'C are calculatedfor Cooma M g ) : 0 . 2 1 ,a : 0 . 1 5 5c m ,. f : 0 . 0 1 3 3 , T : 7 0 0' C l a n d
biotite and Fe-mica biotite, respectively,using Dodson's found that a.nvaied between 0. ll and 0.18 cm (note
(1913) solution of the diflusion equation,the experimen- that the latter value is physically impossible).
tally determined Arrhenius parameters,and assuming a
: 0.015 cm and l0'C/Ma cooling.More complex 40Art! Effect of biotite composition on Ar retentivity
retention properties in biotite indicated by ao[11ts;t luThe remarkable similarity of experimental results obser-spot-profiling studies dictate the use of more general tained for Fe-mica biotite and Cooma biotite casts seridiffusion models such as MP or MDD that are capable ous doubt upon the view that Ar retentivity in biotite
of describing this behavior. Unfortunately these models increasessimply as a function of Fe content,as proposed
introduce additional parameters that are unlikely to be by Norwood (1974) and.Harrison et al. (1985).When the
constrained by aoArl3eArdata becauseof the instability current experimental results are consideredtogether with
of biotite during in vacuo incremental heating experi- those of Hess et al. (1987) and Norwood (1974), it apments.
pears most probable that typical intermediate biotite
As a first approximation, the MP and MDD models compositionssuch as those depictedin Figure I possess
differ only in that interaction is permitted betweenintra- broadly similar aoAr* diffusion properties.
crystalline domains and high-diffusivity pathways in the
Within silicate structures,the diffusion energeticsof a
former but is precluded in the latter. This differencelim- chemically inert substancesuch as Ar are likely to depend
its the predictive value of the MP model for thermo- primarily upon potential energy changesincurred in the
chronology to a significantly greater extent than that of distortion of bonds of adjacent atoms during intracrysthe MDD model. For example, interaction parameters talline diffusion (Sardarov 196l). Note that crystalline
must be constrained for every temperature to apply the defectsand phaseboundarieswith intergrown phasessuch
MP model. Alternatively, all that is required to calculate as chlorite also represent likely sites for Ar residence.
a bulk closure temperature in the MDD model is a valid However, although high-diffusivity pathways related to
estimate of a"o.
the occurrenceof these featurescould significantly facil-
950
GROVE AND HARRISON: rcAr* DIFFUSION IN Fe-RICH BIOTITE
itate 40Ar* transport from biotite (Hess et al. 1987; Lo
and Onstott 1989; Onstott et al. l99l; Lee 1995),the
effectsare unpredictable becauseof uncertainties regarding their volumetric significanceand degreeof interconnectivity. Becauseofits large size,radiogenicAr confined
within homogeneousmica is likely to be confined to vacant interlayer sites between the comparatively closer
packed 2:l layer units. This is demonstratedby the ionic
porosity model, which predicts significantly higher diffusivities in the interlayer region relative to either the
tetrahedralor octahedralsheets(Fortier and Giletti 1989).
Halogens substituted for OH groups may play a significant role in 40Ar* retentivity. Interlayer bonding forces
in biotite are primarily due to electrostaticattraction between uncompensatedchargeson the 2: I layer structures
and the interlayer cations (Bailey 1984). In trioctahedral
micas, the strength of the interlayer bond is appreciably
weakened by the positioning of the OH group (Giese
1984). Becauseoccupation of all three octahedralsites
forcesthe OH group to be oriented essentiallyperpendicular to (001), the exposedproton is situateddirectly above
K+ in the interlayer cavity. K+-H+ repulsion reducesthe
interlayer attraction, increasingthe basal spacingand destabilizing the interlayer cation (Giese 1984).This repulsion is progressivelyeliminated by substitution of F or
Cl- for the OH group with concomitant reduction in cell
parameters (Noda and Ushiro 1964; Takeda and Morosin 1978).Calculationsperformedby Giese(198a) indicate that interlayer bonding energiesincreaseby about 5
kcal/mol or 25o/owith substitution of F- for OH- in
phlogopite. The higher F content of Fe-mica biotite could
explain its slightly higher aoAr* retentivity relative to
Cooma biotite (Table l). Note that becausethe maximum amount of Cl substitution for OH- is small (generally <5-l0o/o) relative to the amount of F- that may
be accommodated (up to 1000/0),F concentration plays
the major role in influencing interlayer bonding in biotite
(Munoz 1984).The apparentincreasein aoAr*diffusivity
with increasing Mg/(Mg + Fe) could be linked to F content. The affinity of F for Mg-rich biotite has been well
documented (Guidotti 1984; Speer 1984). The F-rich
(660/o)phlogopite examined by Giletti (1974) is significantly more retentive of o0Ar* than the intermediate biotite compositionsinvestigatedhere.
AcxNowr,nocMENTs
We thank O.M. Lovera, C.E. Manning, M.T. Heizler, W.A. Dollase,
and P.S. Dahl for helpful discussions We are particularly gateful for
assistanceby R.J. Jonesin electron microprobe measurements,SEM imaging, and determination ofthe cell parametersofthe starting materials.
We thank B.J. Giletti for supplying us with biotite samples studied by
Norwood (1974) for electron microprobe analysis.M. Coscaand L Villa
are acknowledgedfor providing constructive reviews, which improved
the manuscnpt.
RrrnnnNcps crrED
Bailey, S.W. (1984) Crystal chemistry of the true micas. In Mineralogical
Society of America Reviews in Mineralogy, 13, I 3-60.
Brandt, S.B., Smirnov, V.N., Lapides,I.L., Volkova, N.V., and Kovalen-
ko, V.I. (1967) Radiogenic argon as geochemicalindicator of hydrothermal stability of some minerals. Geochemistry International, 4, 826829.
Cebula, G.T., Kunk, M.J., Mehnert, H.H., Naeser, C.W., Obradovich,
J.D., and Sutter,J.F. (1986) The Fish Canyon Tufl, a potential standard
and fission-trackdating methods (abs.).Terra Cognita,
for the aoAr-3eAr
6,139.
Copeland, P., Harrison, T.M., Kidd, W.S F., Ronghua X., and Yuquan,
Z. (1987) Rapid early Miocene accelerationof uplift in the Gangdese
belt, Xizang (southern Tibet), and its bearing on the accommodation
mechanism of the India-Asia collision Earth and Planetary Science
Letters, 86, 240-252.
Crank, J. (1975) The mathematicsof diffi-rsion,414 p. Oxford University
Press,New York.
Dodson, M.H. (1973) Closure temperature in cooling geochronological
and petrological systems.Contributions to Mineralogy and Petrology,
40,259-279.
Foland, K.A. (1974) aoAr diffirsion in homogeneousorthoclase and an
interpretation of Ar difiirsion in K-feldspar. Geochimica et CosmochirnicaActa. 38. l5l-166.
Fortier, S.M., and Giletti, B.J. (1989) An empirical model for predicting
diffirsion coefficientsin silicate minerals. Science,245, l48l-1484.
Frantz,J.D., Popp, R.K., and Boctor, N.Z. (1981) Mineral-solution equilibria: V. Solubilities of rock forming minerals in supercritical fluids.
Geochimica et Cosmochimica Acta, 45, 69-7 8.
Gaber, L.J., Foland, K.A., and Corbato, C.E. (1988) On the significance
ofargon releasefrom biotite and amphibole during {Arl3eAr vacuum
heating. Geochimica et Cosmochimica Acta, 52,2457-2465.
Giese,R.F. (1984) Electrostaticenergymodels of micas. In Mineralogical
Society of America Reviews in Mineralogy, 13, 105-144.
Giletti, B.J (1974) Studies in diffusion: I. Argon in phlogopite mica In
A W. Hofmann, B.J. Giletti, H.S. Yoder, and R.A. Yund, Eds., Geochemical transport and kinetics, p. 107-115. Carnegie Institute of
Washington, publication 634
Goodwin, L.B., and Renne, P.R. (1991) Effectsof progressivemylonitization on Ar retention in biotites from the SantaRosa mylonite zone,
California, and thermochronologicimplications. Contributions to Mineralogy and Petrology, 108, 283-297
Govindaradu, K. (l 979) Report (l 968-1 978) on two mica referencesamples: Biotite Fe-mica and phlogopite mica-Mg. GeostandardsNewsletter.3.3-24.
Grove, M., and Harrison, T.M (1994) Argon loss from F-OH phlogopite.
U.S. GeologicSurveyCircular, 1107, I19.
Guidotti, C.V. (1984) Micas in metamorphic rocks. In Mineralogical Society of America Reviews in Mineralogy, 13,357-468.
Hanson, G.N., and Gast, P.W. (1967) Kinetic studies in contact metamorphic zones.Geochimica et Cosmochimrca Acta, 3 1, I I I 9- I I 53.
Hansen,G.N., Simmons, K.R., and Bence,A.E. (1975)40Ar/3'Aragespectmm ages for biotite, hornblende, and muscovite in a contact metamorphic zone. Geochimica et Cosmochimica Acta, 39, 1269-1277.
Harrison, T.M., Duncan, I, and McDougall, I. (1985) Diffi.rsionof 4Ar
in biotite: Temperature, pressureand compositional effects.Geochimica et cosmochimica Acta, 49,2461-2468.
Harrison, T.M., and Fitz Gerald, J.D. (1986) Exsolution in hornblende
for aArl3eAr agespectraand closuretemperature.
and its consequences
Geochimica et Cosmochimica Acta, 50, 247-253.
Helgeson,H.C., Murphy, W.M., and Aagaard,R. (1984) Thermodynamics and kinetic constraintson reaction rates among minerals and aqueous solutions: II. Rate constants,effectivesurfacearea,and the hydrolysis of feldspar. Geochimica et Cosmochimica Acta, 48, 2405-2432.
Hess,J.C., Lippolt, H.J., and Wirth, R. (1987) Interpretation of 4oAr/reAr
spectraof biotites: Evidencefrom hydrothermal degassingexperiments
and TEM studies ChemicalGeology,66,137-149.
Hess, J.C., Lippolt, H.J., Gurbanov, A.G., and Michalski, I. (1993) The
cooling history of the late Pliocene Eldzhurtinskiy granite (Caucasus,
Russia)and the thermochronologic potential ofgrain sizelagerelationships. Earth and Planetary ScienceIrtters, I 17, 393-406.
Hodges,K.V., Hames, W E , and Bowring, S.A. (1994) &Ar/leAr agegradients in micas from a high-temperature,low-pressuremetamorphic
terrane: Evidence for very slow cooling and implications for the interpretation ofage spectra.Geology, 22,55-58.
GROVE AND HARRISON' 40Ar* DIFFUSION IN Fe-RICH BIOTITE
Huebner, J.S. (1971) Buffering techniquesfor hydrostatic systemsat elevated pressures.In G.C. Ulmer, Ed., Researchtechniquesfor high pressure and high temperature,p.123-178 Springer-Verlag,New York.
Kelley, S.P, Arnaud, NO., Canoll, M.R., and Draper, D.S. (1994a)A
UV laser ablation microprobe technique for argon isotope analysis.
U.S. GeologicSurveyCircular, 1107,166.
Kelley, S.P., Reddy, S.M., and Maddock, R. (1994b) Iaser-probe noAr/
3'Ar investigation ofa pseudotachyliteand its host rock from the outer
Isles thrust, Scotland. Geology, 22,443-446.
Kerrick, D.M., Lasaga,A.C., and Raeburn, S.P. (1991) Kinetic of heterogeneous reactions. In Mineralogical Society of America Reviews in
Mineralogy, 26, 583-67 l.
Lee, J.K W. (1995) Multipath diffusion in geochronology.Contributions
to Mineralogy and Petrology, 120,60-82.
Lee, J.K.W , and Adama, A.A. (1992) Multipath difirsion: A generalnumerical model. Computersand Geosciences,
18, 531-555.
Lo, C H., and Onstott, T C. (1989) 3eArrecoil artifacts in chloritized biotite. Geochimica et Cosmochimica Acta. 53.2697-2711.
Iovera, O.M. (1992) Computer programs to model 40Ar/3eArdiffi.rsion
data from multidomain samples.Computersand Geosciences,18, 7898 13 .
Iovera, O.M., Richter, F.M., and Harrison, T.M. (1989) The @Ar/3,Ar
thermochronometry for slowly cooled sampleshaving a distribution of
domain sizes.Joumal of GeophysicalResearch,94, 179 17-17 935.
McDougall, I., and Harrison, T.M. (1988) Geochronology and thermochronology by the aoArlr,Ar method, 212 p. Oxford Monographs on
Geology and Geophysics,Oxford University Press,New York.
Munoz, J.L. (1984) F-OH and CI-OH exchangein micaswith applications
to hydrothermal ore deposits.In Mineralogical Societyof America Reviews in Mineralogy, 13, 469-494.
Murphy, W M., and Helgeson,H.C. (1989) Thermodynamic and kinetic
constraints on reaction rates among minerals and aqueoussolutions:
IV. Retrieval of rate constantsand activation parametersfor the hydrolysis of pyroxene, wollasotonite, olivine, anadalusite,quartz, and
nepheline.AmericanJoumal ofScience,289, l7-101.
Noda, T., and Ushiro, M. (1964) Hydrothermal synthesisof fluorinehydroxyl-phlogopite: Part IL Relationship between the fluonne content, lattice constants,and the conditions of synthesisof fluorine-hydroxyl-phlogopite. Geochemistry International, I, 96-104.
Norwood, C.B. (1974) Radiogenicargon diffusion in the biotite micas, 58
p. M.S. thesis, Brown University, Providence,Rhode Island.
Onstott, T.C., Phillips, D., and Pringle-Goodell, L. (1991) Laser microprobe measurementofchlorine and argonzonation in biotite. Chemical
G e o l o g y , 9 0 ,1 4 5 - 1 6 8 .
Ozima, M, and Podosek,FA. (1983) Noble gas geochemistry,367 p.
Cambridge University Press,Cambridge.
Phillips, D. (1991) Argon isotope and halogen chemistry of phlogopite
from South African kimberlites: A combined step-heating,laserprobe,
electron microprobe and TEM study. Chemical Geology, 8'l, 7 l-98.
Phillips, D., and Onstott, T.C. (1988) Argon isotopic zoning in mantle
phlogopite. Geology, 16, 542-546.
Reichenberg,D. (1953) Properties ofion-exchange resins in relation to
their structure: IIL Kinetics of exchange.American Chemical Society
Journal,75, 589-597
Roubault, M., de Ia Roche,H., and Govindaraju,IL (1968)Report (19661968) on geochemicalstandards:Granites GR, GA, GH; basalt BR;
ferriferous biotite mica-Fe; phlogopite mica-Mg. Sciencesde la Terre,
Tome XIII. n. 4.379-404.
951
Rutherford, M J. (1973) The phaserelations ofaluminous iron biotites in
the systemKAlSi3O'-KAlSiOo-AlrO,-Fe-O-H.Joumal of Petrology,14,
I 59- I 80.
Sanz,J., Gonzalez-Carreno,T., and Gancedo, R. (1983) On dehydroxylation mechanisms of a biotite in vacuo and in oxygen. Physics and
Chemistryof Minerals,9, 14-18.
Sardarov, S S. (1961) Bond energyand retention of radiogenic argon in
micas. Geochemistry Intemational, l, 33-44.
Speer, J.A. (1984) Micas in igenous rocks. In Mineralogical Society of
America Reviews in Mineralogy, 13,299-356.
Takeda, H., and Morosin, B. (1978) Comparison of observed and predicted structural parametersof mica at high temperature.Acta Crystallographica,B3l, 2444-2452.
Tetley, N.W. (1978) Geochronologyby the 40Arl3'Artechniqueusing HIFAR reactor, 287 p. Ph.D. thesis,Australian National University, Canberra, Australia.
Vedder, W., and Wilkins, R.W.T. (1969) Dehydroxylation and rehydroxylation, oxidation and reduction of micas. American Mineralogist, 54,
482-509
Vernon, R.H. (1988) Sequentialgrowth ofandalusite and cordierite porphyroblasts, Cooma Complex, Australia: Microstructural evidence of
a progradereaction. Journal ofMetamorphic Geology, 6,255-269.
Vilta, I.M., and Puxeddu, M. (1994) Geochronology of the Iarderello
geothermal field: New data and the "closure temperature" issue.Contributions to Mineralogy and Petrology, 115,415-426.
westcotr, M.R. (1966) Ioss of argon from biotite in a thermal metamorphism. Nature, 210, 83-84.
Wones, D R, and Eugster,H.P. (1965) Stability of biotite: Experiment,
theory, and application. American Mineralogist, 50, 1228-1272.
Wood, B.J., and Walther, J.V. (1983) Rates of hydrothermal reactions.
Science.222, 554-555.
Wright, N., Layer, P.W., and York, D. (1991) New insights into thermal
history from single grain 4oAr/reAranalysisof biotite. Earth and Planetary ScienceLetters, 104, 70-79.
Mencn 9, 1995
Mexuscmsr RECETvED
Mnxuscnrpr AccEprEDMmcn 12, 1996
AppENDrxTABLE1.
Compositions of the biotite of Norwood
(19741
Si
r4rAl
t6lAl
Ti
Cr
Fe
Mn
Mg
K
Na
Total Cations
std-s
srd-6
std-8
2.77
1.23
0.19
0.16
0.00
1.11
0.02
1.35
0.94
2.80
1.20
0.28
0.12
0.00
1.29
0.02
1.11
0.96
0.00
7.80
2.79
1.21
0.12
018
0.00
1.08
0.02
1.33
0.95
0.02
7.70
0.o2
7.78
Notej Results of electron microprobeanalysis performed at UCLA with
materials supplied by B.J. Giletti. These compositions differ appreciably
from those reported by Norwood (1974).