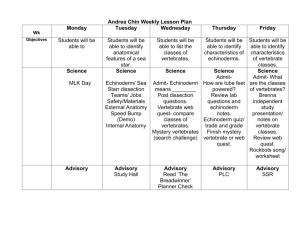
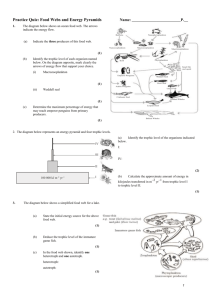
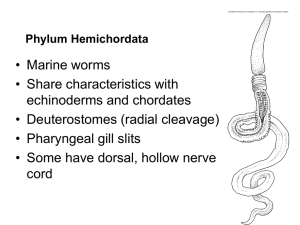
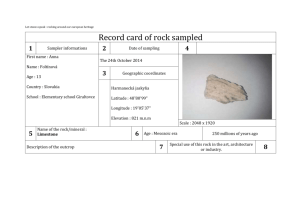
Investigation into echinoderm species richness
advertisement

Investigation into echinoderm species richness and abundance within the rockpool habitats of the Watamu Marine National Park, Kenya Chloe Naylor BSc Biology 2014 Supervisor: Dr. Felix Eigenbrod Word count: 8,636 1 Summary for the Layman Threats made to echinoderms are increasing due to commercial enterprises such as the trade in echinoderms for aquariums and souvenirs. Besides that, the effects of commercial fishing, particularly ‘dredging’ which involves clearing the sea bed, also have negative impacts as some echinoderms are caught as by-catch and their habitats are destroyed. These threats highlight the importance of Marine Parks whose conservation methods are vital in sustaining echinoderm species richness and abundance worldwide. One such park is the Watamu National Marine Park of Kenya. Within the park many echinoderms inhabit, not only the coral reefs but also the rockpools that are located on the rock platforms along the Watamu Marine Park beach. However little research has been conducted to investigate exactly what species of echinoderms live in these habitats or their distribution amongst the platforms. Research of this kind could contribute significantly to the park’s conservation management. This study investigated what echinoderm species could be found amongst these rock platforms and whether their species richness and abundance varied within and between the platforms. 151 transects were conducted on 11 rock platforms along the beach and 5 species of brittlestars, 1 of starfish, 1 of sea cucumber and 4 species of sea urchin were found and it was determined that their abundance and species richness did vary significantly within and between the platforms. The habitat variables that were included in the analysis to determine whether they had an effect on this distribution were rockpool number within the transect, rockpool depths, percentage of rockpools that had living seagrass and the type of substratum found on the rockpool floor. The results were that none of these factors affected echinoderm species richness while only floor substratum type affected total echinoderm abundance. It is likely that there were biotic and abiotic variables that were not measured in this study which were the main factors affecting echinoderm distribution; for instance the tide level at which the platforms were submerged underwater. As well as determining that these habitat factors did not affect echinoderm distribution, the study also concluded that there were rock platform hot spots of relatively high echinoderm species richness. This knowledge as well as the echinoderm data collection should contribute to the database of the Marine Park and aid in conservation management decision making. 2 Abstract The Watamu National Park of Kenya has a series of rock platforms where there are rockpools. These rockpools have had little research on the fauna that inhabit them and so this study aimed to investigate the echinoderm species richness and total abundance in these rockpools. Besides looking at whether these varied within and between rock platforms the habitat variables of rockpool number, rockpool depth, percentage rockpools with living seagrass and rockpool floor substratum type were also compared within the analysis in order to determine whether they were effecting species richness and abundance distribution. 151 transects were sampled across 11 rock platforms and 11 species across 4 echinoderm classes were found. The results of the analysis were that the echinoderm species richness and abundance did vary significantly within and between rock platforms however none of the habitat variables had an effect on this variation with the exception that floor substratum type did have an effect on the distribution of the echinoderm total abundance. When investigating whether the habitat variables had an effect on the distribution of individual echinoderm species it was again found that, on the whole, these habitat variables did affect their distribution. The conclusions of this study were that the habitat variables measured did not affect the distribution of the echinoderm species richness and abundance and that it must be either biotic or abiotic variables that were not measured in the study which are causing the variation within and between the rock platforms. 3 Content 1. Literature Review...................................................................................................6 1.1 Watamu Marine National Park...............................................................................6 1.2 Phylum Echinodermata...........................................................................................6 1.3 Importance of Echinoderms....................................................................................8 1.4 Threats to Echinoderms........................................................................................10 1.5 Studies conducted on Echinoderm Distribution and Abundance.........................11 2. Aims of the project................................................................................................13 3. Materials and Methods.........................................................................................14 3.1 Study sites.............................................................................................................14 3.2 Transect Procedure...............................................................................................15 3.3 Measuring Rockpool Habitat data.........................................................................15 3.4 Finding, Handling and Documenting Echinoderms...............................................16 3.5 Analysis..................................................................................................................17 4. Results..................................................................................................................17 4.1 Echinoderm species found in the study................................................................17 4.2 Echinoderm species richness distribution.............................................................18 4.2.1 General linear model.................................................................................18 4.2.2 Distance class post-hoc analysis................................................................18 4.2.3 Rock platform visual comparison..............................................................19 4.3 Echinoderm abundance distribution.....................................................................21 4.3.1 General linear model.................................................................................21 4.3.2 Distance class post-hoc analysis................................................................22 4.3.3 Rock platform graph analysis....................................................................23 4.3.4 Floor substratum graph analysis...............................................................24 4.4 Effect of habitat variables on individual Echinoderm species distribution...........24 4.4.1 Logistic regression analysis of distribution of species Ophiocoma scolopendrina............................................................................................25 4.4.2 Logistic regression analysis of distribution of species Amphiura dejectoides................................................................................................25 4.4.3 Logistic regression analysis of distribution of species Echinometra mathaei.....................................................................................................26 4.4.4 Logistic regression analysis of distribution of species Echinothrix diadema.....................................................................................................27 4.4.5 Logistic regression analysis of distribution of species Tripneustes gratilla.......................................................................................................28 4.5 Association between Echinothrix diadema and Echinometra mathaei abundance and distribution.....................................................................................................29 5. Discussion.............................................................................................................29 5.1 Echinoderms found in the study...........................................................................29 5.2 Echinoderm species richness and the habitat variables.......................................30 5.3 Echinoderm abundance and the habitat variables...............................................31 5.4 Individual echinoderm species and the habitat variables....................................32 5.5 Echinometra mathaei and Echinothrix diadema...................................................33 5.6 Recommendations for future research and relevancy of study to conservation management.........................................................................................................34 4 6. References............................................................................................................36 7. Appendix..............................................................................................................40 7.1 Study site Locations..............................................................................................40 7.2 Assumptions of general linear model for Echinoderm species richness..............41 7.3 Assumptions of general linear model for Echinoderm abundance......................42 7.4 Assumptions of the Logistic regression analyses.................................................44 7.5 Results of Logistic regression analysis..................................................................47 7.6 Monotopic assumption of Spearman’s rank correlation between Echinothrix diadema and Echinometra mathaei.....................................................................52 5 1. Literature review 1.1 Watamu Marine National Park The original primary objective of marine national parks has been to assist conservation or non-fisheries use of use of an area or resource (Arara and Rose, 2004). Kenya’s 450 km of coastline has several such marine national parks which prohibit fishing and collection of marine organisms (Samoilys, 1988). Often these marine parks focus around coral reefs; within Kenya, the coral reefs are typically shallow mainland fringing reefs, often enclosing a lagoon or moat (Hamilton and Brake, 1984). Easily accessible coral reefs are important to Kenya’s economy as a source of revenue from the tourism industry as approximately 124,000 tourists visit the coast per year (Samoilys, 1988). The Malindi and Watamu Marine Parks are some of these important marine parks located on the Kenyan coast. They were the first Marine National parks to be established in the country in 1968 and they were third in the world (KWS, 2014). The purpose of these parks was to protect biodiversity, manage resources in a sustainable way to protect the livelihoods of coastal communities and manage tourism (Sluka et al, 2012). These two parks were enclosed into the Malindi National Reserve under UNESCO in 1979 although the two parks are maintained separately (KWS, 2014). The reserve encompasses 34 km2 while the Watamu Marine Park is 10 km2 (KWS, 2014). The Marine Park encompasses a complex of marine and tidal habitats including a mangrove forest in a creek characterized by tidal mud flats and a shallow coral reef. The beach adjacent to this coral reef is sandy with large rock outcrops close to the shore, which have multiple rockpools. These rockpools contain a wide range of biota including fish, corals, echinoderms, sponges and other invertebrates. Research is being undergone to provide baseline biodiversity and ecology monitoring in the coral reef and among these rock platforms (Sluka et al, 2012). 1.2 Phylum Echinodermata Echinodermata is a phylum that is classified under the superphylum of Deuterostomia which it shares with Chordata and Hemichodata (Cameron et al, 2000). The phylum comprises of five extant classes of echinoderms; Asteroidea, Ophiuroidea, Echinoidea, Holothuroidea and Crinoidea (Pawson, 2007). There was, at one point, speculation over whether 6 concentricycloids should make up a sixth class (Baker et al, 1986), however due to further morphological, cladistic and molecular evidence, concentricycloids are grouped in the class of Asteroidea (Mah, 2006).The phylum includes approximately 7000 living species and 13,000 fossil species; this group of animals can be briefly defined as containing a calcium carbonate skeleton in the form of calcite, a unique water-vascular system which is used for feeding and locomotion and a five-part radial symmetry (Pawson, 2007). However, there are many exceptions to this general body plan; some taxa of Holothuroidea lack calcite in their walls, some Asteroidea have more than five part radial symmetry (some sea stars have as many as 50 arms) and many echinoderms have superimposed bilateral symmetry on the radial pattern (Pawson, 2007). It has been suggested that the only unifying taxonomic characteristic of the phylum is that all its extant members are marine (Pawson, 2007). The Asteroidea class contains the sea stars of which there are 2100 species inhabiting the oceans today (Pawson, 2007). Sea stars typically have a central disk out of which project five arms which are narrow at the tip and then widens at the base (Barnes, 1980). The mouth is located at the centre of the underside of the disk and from the mouth a wide furrow extends into each arm within which there are two or four rows of small tubular projections or ‘tube feet’ which are used for locomotion (Barnes, 1980). Asteroidea are carnivores that tend to feed on invertebrates, such as bivalves, polychaetes and crustaceans though the particular diet varies among species (Barnes, 1980). The Ophiuroidea is a class that contains basket stars and serpent stars or brittle stars and encompasses 2000 living species (Pawson, 2007). Similar to Asteroidea, Ophuiroids also possess arms however these arms are more sharply set off from the central disk; they appear jointed due to the presence of four longitudinal rows of shields (Barnes, 1980). They also do not use their tube feet for locomotion, instead they use their arms to rapidly crawl and some species can even swim (Barnes, 1980). Ophuiroids are scavengers, deposit feeders or filter feeders; many species use their arms to trap detritus on the mucus strand which run between the spines on the arms that they lift upward and wave about in the water (Barnes, 1980). Echinoidea are made up of 800 species of echinoderms known as sea urchins, heart urchins and sand dollars (Pawson, 2007). Echinoidea, unlike Asteroidea and Ophiuroidea, do not 7 have arms; they have spherical or oval bodies typically covered by movable spines (Barnes, 1980). They are generally adapted to living on rocks or other types of hard bottoms and use their spines to crawl along the ground (Barnes, 1980). Echinoids are generalist grazers which have a mouth apparatus known as an Aristotle’s lantern that is composed of five calcareous plates known as pyramids (Barnes, 1980). The echinoids scrape the substrates on which they live with their Aristotle’s lantern, eating mostly algae but also other plant and animal material they happen to come across; what each species will eat will depend on the area in which they live and what is available (Barnes, 1980). Holothurians number approximately 1400 living species more commonly known as sea cucumbers (Pawson, 2007). Like echinoids, holothurians do not have arms; they are unique in that their polar axis is lengthened giving the body an elongated shape (Barnes, 1980). They are relatively sluggish animals that live on the bottom surface or burrow into mud, those that do move do so with either their tube feet similar to asteroids or by contracting their circular and longitudinal muscles in an action akin to the borrowing of earthworms (Barnes, 1980). Holothurians are chiefly deposit or suspension feeders that use their tentacles around their mouths to sweep over the bottom surface or hold them out in the water to trap particulate matter (Barnes, 1980). The Crinoidea class consist of 650 extant species of feather stars and sea lilies (Pawson, 2007). This investigation will not focus on crinoids as they often live at depths of 100 meters (Barnes, 1980) and so are not relevant to this study which focuses on echinoderms which can be found in rockpools. 1.3 Importance of Echinoderms Echinoderms are an ecologically and economically significant group of invertebrates. They are prime candidates for model toxicological test organisms for marine ecosystems (Zito et al, 2005). This is due to reasons such as their ubiquitous distribution, their susceptibility to micropollutants stored in marine sediments and their sensitivity to many types of contaminants (Micael et al, 2009). The classes of echinoderms are uniquely important to the environment, for instance deposit feeding holothurians are important bioturbators, altering the stratification and stability of muddy and sandy bottoms (Anderson et al, 2011). On coral reefs sea cucumbers can bioturbate the upper 5 mm of sediment once a year (4600 kg dry 8 weight year-1 1000 m-2) significantly reducing microalgal biomass (Uthicke, 1999) and recycling nutrients, thereby maintaining the high productivity of these oligotrophic ecosystems (Uthicke, 2001). Holothurians also serve as prey, particularly for crustaceans, asteroideans and fish (Francour, 1997). In addition to their ecological impact, holothurian fisheries are of great economic and social importance to many coastal communities (Anderson et al, 2011). They form the main source of income in the Solomon Islands (Nash and Ramofafia, 2006) and for 5000 families in Sri Lanka (Dissanayake et al, 2010) as well as representing a large proportion of non-fish marine products from the Maldives (Joseph, 2005). Echinoids also play a major ecological role in coral reefs as their grazing can contribute to the control of benthic community structures (Sammarco, 1982). This control is manifested in several ways including direct alteration and abundance of sessile prey (Sammarco, 1982) and controlling the distribution and abundance of coral spat (Sammarco, 1980). Echinoids are also of economic value as the harvest of the roe or gonads of edible species such as Tripneustes gratilla form a major source of livelihood for the local fishing communities in such countries as Bolinao, Pangasinan and the Philippines (Juinio-Menez et al, 1998). Areas of high abundance of suspension-feeding Ophiuroids are an important link between benthic and pelagic ecosystems (Allen, 1998), in that their feeding behaviour is thought to create a flux of organic matter between these two ecosystems and is, therefore, thought to be a part of the nitrogen and carbon cycles of the coastal ecosystem (Davoult et al, 1992). Asteriods have been documented as Keystone species for various ecosystems, in fact when Paine first coined the term ‘Keystone species’ he was using the example of the Pisaster ochraceus starfish which lives on the Pacific coast of North America and whose predation contributes greatly to the biodiversity of the coastal system (Paine, 1966). Other examples of starfish species performing critical roles include the Acanthaster planci which preys upon stony corals and Charona sp. which preys upon the Acanthaster, thus preserving the balance in coral reef ecosystems (Cristancho & Vining, 2004). 9 1.4 Threats to Echinoderms The collecting of echinoderms for various commercial enterprises has been a growing global threat to the phylum (Micael et al, 2009). Sea urchins and sea cucumbers are under high commercial fishing pressure and aspects of their biology compounds the effects of overexploitation on their wild populations (Micael et al, 2009). These aspects include temporal variability in the population density of sea urchins, sporadic and unpredictable recruitment of juveniles and the uncertainty of reproductive success of both species (Micael et al, 2009). Their sedentary lifestyle and habitat requirements also makes them an easier target and more susceptible to overfishing (Micael et al, 2009). Besides targeted commercial fishing, echinoderms are frequent by-catches from use of general fishing hardware (Micael et al, 2009). Echinoderms are indirectly affected by the impacts of trawls and dredges on the benthic habitat though these effects vary according to the fragility of the habitat and the severity of natural disturbance (Micael et al, 2009). Apart from the direct and indirect effects of fishing, echinoderms are also exploited through the global trade of echinoderms for souvenirs, home aquaria and biomedical products (Micael et al, 2009). Echinoderms occupy 17% of the global trade for aquariums (Wabnitz et al, 2003) and little is known of the full extent of the global use of echinoderms as souvenirs (Micael et al, 2009). To aid conservation, it is recommended that there be an improvement of the collection of echinoderm catch data as well as a development of a global database on the biology, ecology, threats, monitoring and conservation of echinoderm species (Micael et al, 2009). A current topical threat to marine ecosystems and organisms is ocean acidification, brought about through increased ocean CO2 levels due to climate change. This reduction of the ocean’s pH is particularly concerning for organisms with calcium carbonate shells or skeletons which are potentially susceptible to dissolution in acidic waters (Orr et al, 2005). As mentioned earlier in section 1.2, echinoderms have calcium carbonate skeletons and so they fall under this category. It has been concluded that echinoderms are robust to near future ocean acidification as a whole however they are likely to be impacted at the ecosystem level (Dupont et al, 2010). Having said that, it is also concluded that the effect of ocean acidification is species specific and will have worse effects on certain species than it 10 will on others; an example of this is the brittle star Ophiothrix fragilis which is predicted under certain scenarios to go extinct by 2050 due to near future ocean conditions (Dupont et al, 2010). The effects of ocean acidification will also affect specific processes differently between the echinoderm classes, for instance ocean acidification causes high mortality of brittlestar larvae while sea urchin larvae are comparatively robust to the ocean’s drop in pH (Dupont et al, 2008). Evidently the acidification of the ocean may have an effect on echinoderms depending on the species but it will certainly have negative effects on echinoderms in general at the ecosystem level. Other stressors upon echinoderm populations associated with climate change include physical disturbance associated with storm events (which are predicted to increase) (Solomon et al, 2007), rising ocean temperatures, rising sea levels (which are predicted to particularly effect invertebrate nurseries of sea grass beds and mangroves) (Lovelock and Ellison, 2007) and changes in salinity and coastal runoff due to changes in rainfall (Przeslawski et al, 2008). These both directly and indirectly impact echinoderm populations as changing conditions leads to ecosystem community shifts which adversely affect echinoderm biodiversity as well as the biodiversity of other marine invertebrates (Przeslawski et al, 2008). 1.5 Studies conducted on Echinoderm distribution and abundance There have been many studies conducted on echinoderm species spatial distributions and densities as such data provides useful baselines to manage local pollutions in light of the increasing harvest of echinoderms for aquarium trade and souvenirs (see section 1.4) (Guzman and Guevara, 2002). One such studied species is the tropical starfish Oreaster reticulates which inhabits mangroves, lagoons and shallow reef environments (Hendler et al, 1995). High densities of Oreaster r. tended to be found in sea grass areas or on mixed substratum of semi-coarse to fine calcareous sand where there is less run-off from rivers (Guzman and Guevara, 2002). It was also discovered that high densities and high recruitment rates of these starfish were found around the Bocas del Toro archipelago of Panama (Guzman and Guevara, 2002). Considering the fact that only 2.8% of the shallow habitats around this area are protected, it has been recommended that the limits of these 11 protected areas be expanded to include more developed and diverse coral reefs and seagrass habitats (Guzman and Guevara, 2002). Other studies have investigated the distribution and abundance of echinoderms within the larger investigation of benthic communities along tropical subtidal habitats such as the study conducted in Southeastern Brazil (Oigman-Pszczol et al, 2004). It was found that echinoderm abundances were comparatively low compared to the phylums of other invertebrates (Oigman-Pszczol et al, 2004). Sea urchins were the most abundant echinoderm species and the analysis revealed that abundance of the sea urchins varied significantly between sites and depths; their abundance tended to decrease with depth (Oigman-Pszczol et al, 2004). The area under study was suffering from urban growth which could be potentially impacting the marine populations, communities and ecosystems and so it was recommended that the biota of the subtidal zones be monitored with taxonomic precision (Oigman-Pszczol et al, 2004). Studies have also been conducted on echinoderm distribution on a much larger scale such as the one conducted on near-shore rocky habitats in 2010 (Iken et al). The study focused on latitudinal trends in echinoderm species richness and large regional hotspots (Iken et al, 2010). The study was conducted over 76 global-distributed sites with 12 ecoregions and it was found that sample-based species richness was overall low; <1-5 species per site (Iken et al, 2010). Intertidal species richness and abundance was highest in Caribbean ecoregions whose echinoderm assemblages were generally dominated by echinoids (Iken et al, 2010). In contrast, the intertidal ecoregions with high abundance and species richness in the Northeast Pacific were dominated by asteroids and holothurians (Iken et al, 2010). Distinct latitudinal trends were found for intertidal assemblages with greater species richness and abundance at high northern latitudes while no such trends were found for subtidal echinoderm assemblages (Iken et al, 2010). It was also found that latitudinal gradients tended to be superseded by regional hotspots whose assemblages were driven by processes such as evolutionary history and overall productivity (Iken et al, 2010). In this study natural and anthropogenic variables were also found to strongly correlate with the echinoderm assemblages; these included salinity, sea-surface temperature, chlorophyll a, primary productivity, inorganic pollution and nutrient contamination (Iken et al, 2010). 12 Evidently multiple studies have been conducted on echinoderm species distribution and abundance, of which the studies discussed are but a few examples. They have been conducted at scales as specific as a single echinoderm species or as general as the benthic fauna of an entire region. They have been investigations at the small scale of a single beach to large scales that span the entire globe. These assessments in marine systems are important not only from the ecological standpoint but also the public and management standpoints; they create greater understanding for managing marine resource use and identifying conservation priorities (Gray, 1997). 2. Aims of the Study The primary aims of this study were, first to establish what species of Echinoderms were present on these particular platforms and second, to determine what possible habitat variables were affecting the distribution of the Echinoderm species richness and abundance across the rock platforms. Knowing what species are present and where there is higher biodiversity and abundance of these species in the Marine Reserve will be important for conservation management purposes. Determining which factors are contributing towards the diversity and abundance of the Echinoderm organisms will also be important for conservation management. Other secondary aims in the study were to investigate if and how habitat variables were affecting the abundance and distribution of individual Echinoderm species. Also, if there were any species that were observed to occur frequently together during data collection, then one of the aims of the study was to establish whether there is an association of the distribution and abundance between the two species. This will contribute towards determining what factors may contribute to the species distribution and will also have relevancy to the conservation of those particular species. 13 3. Materials and Methods 3.1 Study sites Eleven rock platforms were selected as study sites from among the rock platforms that can be found along the Watamu Marine Reserve Beach between Turtle Bay and Geroda, Kenya (see Appendix 7.1). The study sites were selected based upon whether they were close enough to the shore that, during the low tides, the sides closest the land were in contact with the beach and there was very little or no body of water between the rock platform and the beach. This was to ensure that the study site could be categorized into the three distance classes of ‘flanking the sea’ or ‘sea’, ‘flanking the beach’ or ‘land’ and ‘the centre of the platform between the distance classes ‘sea’ and ‘land’’ or ‘top’. Data collection at these study sites were conducted between the 15 th of August and the 15th of September 2013. Data collection took place at the low tides of which the time of day and the length of time of low tide varied from day to day. Data collection was not conducted at night as the darkness would have made it harder to locate and identify Echinoderms and it may have caused a change in the echinoderms behaviour and distribution compared to the daytime. Belt transects were used to sample the study sites and though the number of belt transects varied between study sites due to the variation in size of the platforms however the number of transects tended to vary between 4 and 8 transects per distance class of the rock platform. Figure 1. A sample picture of some of the rockpools found on one of the rock platforms that were researched in this investigation. The picture was taken August 2013 by Chloe Naylor. 14 3.2 Transect procedure The transects were set up using a 100 m long measuring tape which was laid along the rock platform. The starting point of the platform was always at the western end and the data collection always began on the distance class categorized as ‘sea’. 10 m of tape would be laid out at a 90° angle to the sea and then a meter long pole would be used to determine the width of the transect as the person conducting the data collection would walk down the measuring tape holding out the pole so that one end would be over the tape and the other would be facing the sea. Using this pole to judge what rockpools came within the transect, the echinoderm species and the number of individuals within that species within the rockpools would then be counted. In total, 151 transects were conducted on the 11 study site rock platforms. What was considered a rock pool was a depression within the rock platform which had a volume of water within that depression. Sometimes rockpools would be connected together but there would be two distinct depressions within the rock platform although they may have shared the same volume of water. If that were the case then those two depressions would be counted as two separated rockpools as they had two different deepest volumes. A rockpool that counted as being within the transect was any that had come within the metal pole; even those that were not completely within the transect were counted, although only those echinoderms within the transect boundary were counted; those that were found in rockpools which were partly found within the transect but the echinoderms themselves which were not physically within the transect were not counted. That being said, the echinoderm species which were seen on the rock platforms but were not found within the transects were noted down. 3.3 Measuring rockpool habitat data As well as counting the echinoderm species and the number of individuals, habitat data was also taken into account. The depths of the rockpools that were found within the transects were measured using a reed pole that had lines carved into the pole every 10 cm so that the pools estimated depths could be taken as 0-10cm, 10-20cm, etc. Each pool was consistently measured at its deepest point, even those that were only partly found within the transects. As well as pool depth, sea grass coverage was also measured using a number system to 15 judge and estimate the amount of sea grass coverage per pool. If the pool was ¼ covered by sea grass then it received the number 1; if it covered up to half of the pool it was given the number 2 and if it was over half it was given the number 3. This system was decided as it was difficult to judge how much of the pool was covered by sea grass above that of ½ the pool. The percentages of rockpools that had living seagrass in the transects were then calculated from this data. As well as this, the number of rockpools per transect were counted including those that were only partly found within the transects. Typically combinations of sand, rock rubble and/or dead seagrass consisted as the substratum of the rockpools and so four categories of floor substratum were devised: sand, sand and dead seagrass, sand and rock rubble, and sand, rock rubble and dead seagrass. It was recorded which of the categories the floor substratums belonged to in each of the rockpools within the transects. 3.4 Finding, handling and documenting echinoderms If a new species of echinoderm was found then the individual would be placed in a white tub and a picture would be taken of the specimen for later identification. There were individuals that were too difficult to take a picture of, for example there were brittle star species that tended to be found in crevices and therefore were difficult to pull out in order to be placed in the white tub. Each rock pool within the transect was carefully inspected in order to observe any brittlestars which tended to protrude their tentacles out of the crevices when the tide started to come back in. If the rock pool was completely covered in a thick layer of dead seagrass then it was difficult to observe any brittlestar species. This hindered data collection but not to a significant extent as it would be unusually for brittle stars to be in those rockpools as they are filter feeders (Barnes, 1980) and preferred rockpools with an unobstructed flow of water at high tide. In order to determine whether any sea urchins were under the layer of dead sea grass within the rock pools, a wooden pole would be used to move the dead sea grass from side to side in order to get a better view of the rock pool bottom. As well as this, the wooden pole would be pulled along the rockpool bottom in order to feel whether there were any urchins on the bottom of the pool. The dead seagrass would also be searched through by hand as sometimes juvenile urchins could be found sticking to the strands of sea grass. 16 3.5 Analysis The data was handled using Microsoft Excel 2007 and were analysed using IBM SPSS Statistics 21. The data was determined to be normally distributed using visual inspection of their bar charts and Q-Q normal plots and then they were determined to be homogenously distributed with visual inspection of the scatterplots. General linear models were then run on the echinoderm data and the rockpool habitat data. Logistic regression analyses for individual echinoderm species’ data and the habitat data sets. Finally a spearman’s rank correlation was used to investigate an association between the abundance and distribution data of two species of echinoids. 4. Results 4.1 Echinoderm species found in the study Within this study 5 species of Ophuiroidea, 1 species of Asteroidea, 1 species of Holothuroidea and 4 species of Echinoidea were found. Ophuiroidea, therefore, was the most diverse echinoderm class found during the investigation followed by Echinoidea. The most highly abundance species was Echinomertra mathaei and then Ohiphiocoma scolopendrina and Echinodthrix diadema. The most highly distributed echinoderm species was Ophiocoma scolopendrina followed by Echinometra mathaei and Echinothrix diadema (see Table 1). Outside of the transects other species of echinoderms, the starfish Protoreaster lincki and Nardoa variolata, were also discovered on the study sites, however they were not included in the analysis of this study. This indicates that the methods of this investigation did not sample all the echinoderm species that inhabit the rockpools of the study sites. Table 1. The list of the echinoderm species found in the study with the total abundances and how many rock platforms they were found on. Echinoderm Family Ophuiroidea Asteroidea Holothuroidea Echinoderm species Ophiocoma scolopendrina Amphiura dejectoides Ophiomastix venosa Unidentified species 1 Unidentified species 2 Monachaster sanderi Actinopyga Number of rock platforms on which it was found 10 17 Total abundance 268 3 2 2 41 7 4 1 2 1 1 1 1 Echinoidea mauritiana Echinometra mathaei Echinothrix diadema Tripneustes gratilla Diadema setosum 6 5 4 1 275 161 11 1 4.2 Echinoderm species richness distribution 4.2.1. General linear model To understand which, if any, of the independent abiotic factors measured were affecting the Echinoderm phylum distribution amongst the rockpools of the study sites, a general linear model was conducted. This model investigated whether there was a statistically significant difference between the Echinoderm species richness adjusted means of the independent variable groupings; distance class, rock platform, rockpool number, percentage of live seagrass coverage, rockpool depth and rockpool floor substratum type. The species richness data were determined to be normally distributed through visual inspection of its histogram and normal Q-Q plot (see Appendix 7.2 figures 15 and 16) and they were determined to have homogenous variance through visual inspection of the scatterplot of predicted values and standardized residuals (see Appendix 7.2 figure 17). The results of the General Linear model were that there was statistical significance difference in echinoderm species richness between the rock platform distance classes, F(2,129) = 11.374 (p<0.001) partial Ƞ2 = 0.150 and between the different rock platforms, F(10, 129) = 4.112 (p<0.001) partial Ƞ2 = 0.242. There was no statistical significance difference in echinoderm species richness between the floor substratum types, rockpool numbers, percentages of rockpools with living seagrass, or rockpool depths. 4.2.2 Distance class post hoc analysis To investigate how species richness differs between the distance class groupings, post-hoc pair-wise comparison analysis that included a Bonderroni adjustment was conducted. The results of the analysis indicates that the mean echinoderm species richness of distance class ‘land’ was significantly lower than that of distance class ‘sea’ (p<0.001) and significantly lower than that of distance class ‘top’ (p=0.002). However there was no significant difference between the echinoderm species richness of classes ‘sea’ and ‘top’ (p= 0.203) despite the visual observation of the ‘top’ echinoderm species richness mean being lower than that of ‘sea’ (see figure 2). 18 2 Mean echinoderm species number 1.8 1.6 1.4 1.2 1 0.8 0.6 0.4 0.2 0 sea top land Distance class Figure 2. The mean echinoderm species number of the distance classes across all the rock platforms. 4.2.3 Rock platform graph analysis As stated in section 4.1.1, the Echinoderm species richness means were, in general, statistically different between the different rock platforms. Figure 3 indicates that the rock platforms with the largest mean echinoderm species richness were Geroda Rock 1 and 2, Wishing Rock 1 and 2 and Hemmingways Rock 1. Those with the lowest average number of echinoderm species were Turtle Bay Rock 1 and 3, Wishing Rock 3 and Geroda Rock 3. This is confirmed by figures 4 and 5 which give a visual indication as to where each species was found on the study sites along the beach. 19 Mean echinoderm species number 1.8 1.6 1.4 1.2 1 0.8 0.6 0.4 0.2 0 Rock platforms Figure 3. Mean echinoderm species number of each rock platform. Figure 4. Map indicating the locations of the echinoderm species found at the study sites (between Wishing Rock 1 and Hemmingways Rock 1). The locations of the echinoderm 20 markers on the rock platforms are not representative of where on the rock platforms these echinoderm species were found. Figure 5. Map indicating the locations of the echinoderm species found in this study at the platform level (between Geroda Rock 1 and Geroda Rock 3). Similar to Figure C, the locations of the echinoderm markers on the rock platforms are not representative of where on the rock platforms these echinoderm species were found. 4.3 Echinoderm abundance distribution 4.3.1 General linear model Similar to the general linear model conducted in section 4.1.1, this model was using the same independent variable groupings to discover whether there is a statistically significant difference between the mean abundances of the total Echinoderm organism counts. Standardized residuals of the total number of echinoderm organism were seen to not be normally distributed through the visual inspection of the bar chart (see Appendix 7.3 figure 18). As a result of this, the data was transformed to the square-root and the subsequent residuals of the transformed data were shown to be normally distributed through the visual inspection of the bar chart (see Appendix 7.3 figure 19) and the normal Q-Q plot (Appendix 7.3 figure 20). The data was also shown to be relatively but not completely, homogenously 21 varied through visual inspection of the scatter-plot of the predicted values and standardized residuals (see Appendix 7.3 figure 21) however due to the robustness of the model analysis and the high number of data points, it was decided that the test could be attempted despite this, although its results should be interpreted with caution. The results of the general linear model were that the difference between the numbers of echinoderm organisms were statistically significant across the distance classes F(2,129) = 15.44 (p<0.001) partial Ƞ2 = 0.193, across the different rock platforms F(10,129) = 5.239 (p<0.001) partial Ƞ2 = 0.289 and across the different floor substratum types F(2,129) = 2.986 (p=0.034) partial Ƞ2 = 0.065. There was not a statistically significant difference between the total echinoderm numbers in relation to rockpool number, percentage of rockpools with living seagrass and rockpool depths. 4.3.2 Distance class post hoc analysis Post hoc pair-wise comparison analysis with a Bonferroni adjustment of the distance class variable revealed that the difference in the echinoderm organism count was significant between each of the three categories (p= 0.001, p<0.001, 0=0.029). As can be seen from figure 6, the ‘sea’ class had significantly more echinoderm organisms than classes ‘top’ and ‘land’ while ‘top’ had a significantly higher number of echinoderm organisms than ‘land’. Mean total echinoderm organism count (Squareroot transformation) 5 4.5 4 3.5 3 2.5 2 1.5 1 0.5 0 sea top land Distance classes Figure 6. A bar-chart showing the average echinoderm organism counts across the three distance classes. 22 4.3.3 Rock platform graph analysis Figure 7 shows that the rock platforms with the highest total number of echinoderm organisms were Wishing Rock 1 and 2 and Geroda Rock 1 and 2. This means that not only do Wishing Rock 1 and 2 and Geroda Rock 1 and 2 have the high echinoderm species richness but it also has high abundances of these echinoderms. Geroda Rock 2 though it was found to have relatively low species richness; when it is compared to Geroda Rock 1 and Wishing Rocks 1 and 2, it was found to have the highest echinoderm abundance of all the rock platforms. This indicates that though Geroda Rock 2 may not have conditions which allow a high variety of these species to exist, of what species that do manage to survive, the conditions enable them to live in high numbers. Hemmingways Rock 1 on the other hand, though shown to have high species richness, did not have relatively high abundances of the echinoderms when compared to the other rock platforms. Turtle Bay Rock 3, Wishing Rock 3 and Geroda Rock 3 have been consistently found to be low in both echinoderm species richness and abundance. No species and therefore no abundances were found on Turtle Bay Mean total number of echinoderm organisms (squareroot transformation) Rock 1. 6 5 4 3 2 1 0 Rock platform Figure 7. A bar-chart of the mean, total echinoderm organism count per rock platform. 23 4.3.4 Floor substratum type graph analysis As was previously stated in section 4.2.1, the variable of the rockpool floor substratum type was shown to have a significant effect on the number of echinoderm organisms in the rockpools. Figure 8 illustrates that the floor substratum type that had, on average, the highest number of echinoderm organisms was that of the sand, rock rubble and dead Mean echinoderm organism count (squareroot transformation) seagrass while that which had the lowest was sand and dead sea grass. 6 5 4 3 2 1 0 sand sand and dead seagrass sand and rock rubble sand, rock rubble and dead seagrass Floor substratum category Figure 8. A chart of the mean of the total echinoderm organism count for the different floor substratum types of the rockpools. 4.4 Effect of habitat variables on individual Echinoderm species distribution Having established what effects these habitat variables have on the species richness and abundance of Echinoderms as a phylum, it then would be worthwhile to deduce what effects these habitat variables will have on the distribution of individual species. Logistic regression analysis was preformed to ascertain the effects of rockpool umber, percentage of rockpools with living seagrass, rockpool depth and rockpool floor substratum on the likelihood that a certain species of echinoderm will be present in a transect. 24 All species data that was put through the linear regression analysis met the assumption of the continuous independent variables being linearly related to the logit of the dependent variables (see Appendix 7.4 ,Tables 2-6). 4.4.1 Logistic regression analysis of distribution of species Ophiocoma scolopendrina Logistic regression analysis was preformed investigating whether these factors had an effect on the presence or absence of the brittlestar species Ophiocoma scolopendrina (see figure 9) which was one of the most widely distributed species that was found on the study sites (see figures 4 and 5). The logistic regression model for this species was a good fit as was indicated by the statistically insignificant results of the Hosmer and Lemeshow test; X2 = 7.082, df = 8, p=0.528. The model explained 12.5% (Nagelkerke R2) of the variance in the distribution of Ophiocoma scolopendrina and correctly classified 68.2% of transects. Sensitivity of the model was 19.1%, specificity was 90.4%, positive predictive value was 47.4% and negative predictive value was 71.2%. The model showed that none of the predictor variables were statistically significant in determining the presence or absence of Ophiocoma scolopendrina (see Appendix 7.5 table 7). Figure 9. Photo of Ophiocoma scolopendrina taken by Chloe Naylor August 2013 4.4.2 Logistic regression analysis of distribution of species Amphiura dejectoides Logistic regression analysis was also used to investigate whether these factors have an effect on the distribution of Amphiura dejectoides (see figure 10), another brittlestar species 25 commonly found on the rock platforms that were studied in this investigation (see figure C and D).This logistic regression model was a good fit in predicting the distribution of the species as is shown by the Hosmer and Lemeshow test; X2 = 3.921, df = 8, p=0.864. The model explained 24.6% of the variance of the Amphiura dejectoides (Nagelkerke R2) distribution and correctly classified 93.4% of transects. Sensitivity of the model was 0.1%, specificity was 100%, the positive predictive value was 100% and the negative predictive value was 93.3%. Of the predictor variables, rockpool number was the only variable to have a statistically significant effect on the distribution of Amphiura dejectoides, Wald = 4.269, df = 1, sig = 0.039. Increasing the rockpool number by 1 would increase in the likelihood of finding Amphiura dejectoides in that transect 1.084 times (see Appendix 7.5, table 8). Figure 10. Photo of Amphiura dejectoides taken by Chloe Naylor August 2013 4.4.3 Logistic regression analysis of distribution of species Echinometra mathaei The next species whose distribution was compared to the independent variables was Echinometra mathaei (see figure 11), a species of sea urchin which was common and widely distributed among the study sites (see figures 4 and 5). The model is a good fit to the distribution of Echinometra mathaei as shown by the results of the Hosmer and Lemeshow test; X2 = 10.443, df = 8, p = 0.235. The model explained 14.8% (Nagelkerke R2) of the variance in the distribution of the species and correctly classified 86.1% of transects. The sensitivity of the model was 8.7%, its specificity was 100%, the positive predictive value was 100% and the negative predictive value was 85.9%. The model indicates that none of the 26 predictive values are statistically significant in predicting the presence or absence of Echinometra mathaei (see Appendix 7.5 table 9). Figure 11. Photo of Echinometra mathaei taken by Chloe Naylor August 2013 4.4.4 Logistic regression analysis of distribution of species Echinothrix diadema The next species to be analysed with logistic regression was Echinothrix diadema (see figure 12), another species of sea urchin that was commonly found at the study sites. The results of the Hosmer and Lemeshow test indicate that the model of the regression was a good fit, X2 = 2.047, df = 8, p = 0.980. The model explained 16.6% (Nagelkerke R2) of the variance in the Echinothrix diadema distribution and correctly classified 90.7% of transects. Sensitivity of the model was 0%, specificity was 100%, the positive predictive value was 0% and the negative predictive value was 90.7%. The model shows that none of the predictive values are statistically significant in predicting the presence or absence of Echinothrix diadema (see Appendix 7.5 table 10). 27 Figure 12. Photo of Echinothrix diadema taken by Chloe Naylor August 2013 4.4.5 Logistic regression analysis of distribution of species Tripneustes gratilla The final logistic analysis was conducted on another species of sea urchin, Tripneustes gratilla (see figure 13), which was not commonly found on the study sites but when it was observed, it tended to be observed in rockpools with high percentage coverage of living seagrass. The model was a good fit as was shown by the results of the Hosmer and Lemeshow test; X2 = 4.830, df = 8, p = 0.776. The model explained 10.3% of the variance in the Tripneustes gratilla distribution and correctly classified 94% of transects. The sensitivity of the model was 0%, specificity was 100%, the positive predictive value was 0% and the negative predictive value was 94%. The model shows that none of the predictive values are statistically significant in predicting the presence or absence of Tripneustes gratilla (see Appendix 7.5 table 11). The other species recorded in this study were found too rarely to make do a logistic regression analysis. 28 Figure 13. Photo of Tripneustes gratilla taken by Chloe Naylor August 2013 4.5 Association between Echinothrix diadema and Echinometra mathaei abundance and distribution It was observed that Echinothrix diadema and Echinometra mathaei were often found together and when they were found together they tended to occur in high abundances. A spearman’s rank correlation test was implemented to see whether there was an association between these two species’ abundances. The abundance data was transformed using natural log to meet the assumption of the data being monotopic (see Appendix 7.6). Once the test was run it was found that there was a strong positive correlation between the abundance distribution of Echinothrix diadema and that of Echinometra mathaei, rs(11) = 0.731, p = 0.005. 5. Discussion 5.1 Echinoderms found in the study The results of this study indicated that the most abundant echinoderm to be found on the rock platforms of the Watamu Marine Park beach was Echinometra mathaei. While other studies of echinoderms have not found Echinometra m. to be the most abundant of all echinoderm species, they have found that Echinometra m. is the dominant echinoid on the Diani Beach in Kenya (Khamala, 1971). This perhaps is due to the prime environment that is 29 ideal for the Echinometra m. to thrive or it may be a robust species that can adapt to a range of environmental factors. The most diverse echinoderm class was Ophuiroidea which may be representative to how well adapted the class is to exploiting the rockpool type of habitat. Other studies have revealed that the intertidal zone makes a favourable habitat for Ophuiroids as it affords the ability to exploit a nutrient-rich water surface and provides them protection from predators such as fish which are excluded from these platforms at low tide (Oak and Scheibling, 2006).The Asteroidea and Holothuroidea may not have been so diverse and abundant due to the fact that they are mainly shallow water species and so only a relatively few individuals of the larger population living on the coral reefs would occupy the rocky habitats at the fringes of where the majority of these organisms live (Barnes, 1980). 5.2 Echinoderm species richness and the habitat variables According to the general linear model that was conducted in this study, species richness is only significantly different across the distance classes and the rock platforms themselves (see section 4.2.1). The species richness of the echinoderms did not vary significantly between different rockpool numbers, rockpool depths, percentage of rockpools with living sea grass or floor substratum types. That being the case, it is likely that other factors that were not measured during the study, either abiotic or biotic which causes there to be a variation between the species richness of the echinoderms of the different rock platforms. A highly possible variable that was not measured was the amount of time that the rock platforms are submerged during high tide. Though this variable was not measured directly, it was noted during data collection that the rock platforms were exposed at different levels of low tide. Other studies have found that fluctuating levels of salinity have an effect on echinoderm diversity, abundance and population structure and that this sensitivity can be explained by the high permeability of their outer surfaces (Drouin et al, 1985). The amount of time that the rockpools are submerged and exposed would affect salinity levels of the rockpools and so it likely that those rockpools that are closer to land and are exposed for longer periods of time would have higher salinity fluctuations and therefore a lower diversity and abundance of echinoderms. This concurs with what was found in this study as, according to the result of the post-hoc analysis, there was no significant difference between 30 the echinoderm species richness between that the of the ‘sea’ and ‘top’ distance classes however there was a significant difference between ‘land’ and the these other two distance classes (see section 4.2.2). Other variables may also be related to this variation in echinoderm diversity such as the availability of food resources. The land section is exposed more frequently than the ‘top’ and ‘sea’ distance classes and it is less likely that the algal growth will live on the section of the rock platform closest to land as that will be submerged the least amount of time; this will mean it is will be less likely to find echinoid species on this distance class as they are fairly sedentary grazers which scrape algae off the rocks (Barnes, 180). Similarly, ophuiroids are filter feeders and are more likely to inhabit rockpools closer to the seaward side of the rock platforms in order to be nearest to the source of influx of organic matter which they filter out of the seawater with their tentacles (Barnes, 1980). Holothuroids are could be predictable found where they can filter organic materials out of the sediment (Barnes, 180) and that sediment will be more likely to be found on the seaward side of the rock platforms. The asteroids are predators (Barnes, 180) and could predictably be found where their prey is located and so are more likely to be found where the other echinoderms are located which has been established to be on the ‘top’ and ‘sea’ distance classes. The fact that species richness is not significantly different between the distance classes of ‘sea’ and ‘top’ mean abiotic and biotic variables of these two distance classes are similar enough to allow for the same variety of echinoderms to inhabit their rockpools. 5.3 Echinoderm abundance and the habitat variables Similar to the results of species richness, it was found that echinoderm abundance varied significantly between the distance classes and the rock platforms (see section 4.3.1). It is likely that it is for the same reasons of the unmeasured abiotic and biotic variables that were not measured in this study, such length of time the habitats are submerged or availability of food, which are affecting the distribution of the echinoderm numbers along the study sites. The post-hoc analysis conducted on the distance classes revealed that there was a significant difference between the abundance of echinoderms across all three distance classes (see section 4.3.2). This indicates that while, the abiotic and biotic variables were similar enough between the ‘sea’ and ‘top’ distance classes to allow for the same variety of echinoderm species to inhabit their rockpools, the differences between them are 31 enough to mean that there is a higher abundance of echinoderm organisms found on the distance class closest to the sea. This could mean that a biotic variable such as availability of food occurs for a wide variety of echinoderm species across both the ‘top’ and ‘sea’ distance classes however; the availability of food is greater within the ‘sea’ distance classes allowing for a greater abundance of echinoderm organisms. Unlike the results investigating echinoderm species richness however, the results of the analysis showed that the variable of floor substratum types does have an effect on the distribution of total number of echinoderm organisms, though the level of its significance was not as high as that of distance class and rock platforms (see section 4.3.1). The evidence from figure 8 indicates that the floor substratum type which allowed for the highest number of echinoderms was sand, dead seagrass and rock rubble. This is interesting as many studies have found that a positive correlation between habitat heterogeneity and diversity (Tews et al, 2004) which predicts that a more complex rockpool floor substratum would mean greater species richness but not necessarily a greater number of echinoderm organisms. Also figure 8 indicated that the floor substratum type that had the least abundance was sand and dead seagrass rather than just sand. This could indicate that dead seagrass has a detrimental effect on the number of echinoderms that can live within the rockpools. This would be logical for the brittlestar species as the dead seagrass would inhibit their ability to filter feed however, one would not have thought that it would have a detrimental effect on the other classes of echinoderms. Besides this, it was found that sand and rock rubble was floor substratum type with the second highest number of echinoderms; this could indicate that the presence of rock rubble is what causes there to be a higher number of echinoderm organisms. This would be worth investigating in further study. 5.4 Individual species and the habitat variables According to the results of this study, none of the habitat variables measured had an effect on the distribution of the individual echinoderm species Ophiocoma scolopendrina, Amphiura dejectoides, Echinometra mathaei, Echinothrix diadema, and Tripneustes gratilla (see section 4.4). There was one exception in that the results predicted that the likelihood of finding Amphuira d. in a transect was predicted to increase 1.084 times with an increase of one rockpool. This could mean that there are species-specific abiotic or biotic variables that 32 were not measured in the study that predict the distribution of individual echinoderm species and that it is impossible to use generalized habitat variables in order to predict their individual distribution. For instance, a study conducted on Ophiocoma scolopendrina determined that its feeding behaviour (surface-film feeding) is regulated by the tidal cycle and is triggered by suspended particles (Oak and Scheibling, 2006). In order to optimize their ability to obtain organic matter from filtering the seawater, it is likely that they will be located in rockpools that are closest to the water of the rising tide that covers the rock platforms. The abiotic variable that could potentially best predict their distribution would be the amount of time it takes for the water of the rising tide to reach the pool. This would be completely different to what best predicts the distribution of sea urchins which do not filter feed but tend to scrape the surface of substrates. Within one species there can also be variations of habitat preferences which could complicate what factors influence the species distribution. Echinometra mathaei, for instance shows great colour variation and it has be demonstrated in previous studies that these morphological variants have different habitat preferences; for instance one type preferred protected tidepools with rock rubble floor substratums while another type preferred intertidal areas exposed to wave action and which had deep burrows (Nishihara et al, 1991). Clearly not only the variation between the lifestyles of different echinoderm species but also possibly within echinoderm species may mean that shared abiotic variables will not be able to predict individual echinoderm species distributions. 5.5 Echinometra mathaei and Echinothrix diadema The distribution of the abundance of Echinometra m. was found to be strongly associated with that of Echinothrix d (see section 4.5). This could be put down to the similar lifestyles that these two echinoids have; they are both omnivorous grazers that scrape algae and other organic matter of the rock substratum (Barnes, 1980). The fact that they occur in high abundances together is an indication that in those particular areas the conditions are favourable for high number of organisms with that particular lifestyle. However they cannot share the exact same niche as it would be predicted that the better adapted of the two sea urchin species would have outcompeted the other sea urchin species and prevented it from occupying the same habitat. That being the case, the two species have similar enough life 33 styles that they occur in high abundances in the same favourable conditions however their niches are dissimilar enough to allow them to cohabitate the same rock platforms. 5.6 Recommendations for future research and relevancy of study to conservation management This study established that the habitat variables measured had little effect on the distribution of the echinoderm abundance and species richness though it did determine that they varied significantly between the distance classes of the rock platforms and the rock platforms themselves. With this in mind, it might be worthwhile to investigate whether biotic factors such as food availability or presence of non-echinoderm predators have a greater effect on the distribution of the species richness and abundance of the echinoderms. Other abiotic factors that could not be measured in this study due to lack of materials and time but would be worth investigating in future studies were salinity and temperature variation among the rockpools and the amount of time that the rock platforms are exposed at the different tide levels. As well as this there may be anthropogenic effects on the distribution of the echinoderms which could not be measured in this study such as the possibility of echinoderms being illegally collected by tourists and souvenir sellers along the beach. This study established that rockpool floor substratum types did have an effect on the distribution of the echinoderm abundances. A worthwhile further study to this may be to conduct a comparative investigation between the floor substratum types in order to determine whether it is the presence of rock rubble which causes there to be a greater abundance of echinoderms and whether the presence of dead seagrass causes there to be a reduction in the number of echinoderms that can live in the rockpools. This study also established that generalized habitat variables do not predict the distribution of individual echinoderm species and so, other studies would need to conduct more speciesspecific surveys in order to determine what are the most significant factors affecting the distribution and abundance of the individual echinoderm species among the rock platforms. This study determined that the rock platforms investigated that had the greatest echinoderm species richness were Geroda Rock 1 and 2, Wishing Rock 1 and 2 and 34 Hemmingways Rock 1 (see section 4.2.3). These then could be considered rock platform hotspots for echinoderm diversity and this should be considered when planning future conservation management. It may be prudent to enforce greater protection for those particular areas so there are fewer disturbances to the echinoderm populations in order to maintain their high biodiversity. It also determined that some of the rarer echinoderms to be found on the rock platforms belonged to the Asteriodea and Holothuroidea classes which could mean that they should be carefully monitored as their relatively lower populations would be less resistant to disturbances to the rockpool ecosystems. 6. References 35 Allen, J.R. (1998) Suspension feeding in Ophiothrix fragilis: efficiency of particle retention and implications for the use of encounter rate models. Marine Biology, 132: 383-390. Anderson, S.C., Flemming, J.M., Watson R. & Lotze, H.K. (2011) Serial exploitation of global sea cucumber fisheries. Fish and Fisheries, 12: 317-339. Baker, A.N., Rowe, F.W.E. & Clark, H.E.S. (1986) A new class of Echinodermata from New Zealand. Nature, 321: 862–864. Barnes, R.D. (1980) Invertebrate Zoology Fourth Edition. Holt-Saunders Japan, Tokyo. Cameron, C.B., Garey, J.R. & Swalla, B.J. (2000) Evolution of the chordate body plan: new insights from phylogenetic analyses of deuterostome phyla. Proceedings of the National Academy of Sciences USA, 97: 4469–4474. Christancho, S. & Vining, J. (2004) Culturally defined Keystone Species. Human Ecology Review, 11(2): 153-164. Davoult, D., Degros, N., Janquin, M.A., & Soyez, B. (1992) Biometrics, carbon and nitrogen content in the Ophiuroid Ophiothrix fragilis. J. Mar. boil. Ass. U.K, 72: 915-918. Dissanayake, D., Athukorala, S. & Amarasiri, C. (2010) Present status of the sea cucumber fishery in Sri Lanka. SPC Beche-de-mer Information Bulletin, 30: 14–20. Drouin, G., Himmelman, J. H., Beland, P. (1985) Impact of tidal salinity fluctuations on echinoderm and mollusc populations. Canadian Journal of Zoology, 63(6): 1377-1387 Dupont S., Havenhand J., Thorndyke W., Peck L., & Thorndyke M. (2008) CO2-driven ocean acidification radically affect larval survival and development in the brittlestar Ophiothrix fragilis. Mar Ecol Prog Ser, 373:285–294. Dupont S., Ortega-Martinez, O., & Thorndyke, M. (2010) Impact of near-future ocean acidification on echinoderms. Etoxicology, 19: 449-462. Francour, P. (1997) Predation on holothurians: a literature review. Invertebrate Biology, 116: 52–60. Gray, J.S. (1997) Marine biodiversity: patterns, threats and conservation needs. Biodiversity Conservation, 6: 153–175. Guzman, H.M. & Guevara, C.A. (2002) Annual reproductive cycle, spatial distribution, abundance, and size structure of Oreaster reticulatus (Echinodermata: Asteroidea) in Bocas del Toro, Panama. Marine Biology, 141: 1077-1084. Hamilton, H.G.H & Brakel, W.H. 1984. Structure and coral fauna of east African reefs. Bull. tJar. Sci. 34(2): 248-266. Hendler G., Miller J.E., Pawson D.L., Kier P.M. (1995) Echinoderms of Florida and the Caribbean. Sea stars, sea urchins and allies. Smithsonian Institution Press, Washington, D.C. 36 Iken, K., Konar, B., Benedetti-Cecchi, L., Cruz-Motta, J.J., Knowlton, A., Pohle, G., Mead, A., Miloslavich, P., Wong, M., Trott, T., Mieszkowska, N., Riosmena-Rodriguez, Airoldi, L., Kimani, E., Shirayama, Y., Fraschetti, S., Ortiz-Touzet & Sily, A. (2010) Large-Scale Spatial Distribution Patterns of Echinoderms in Nearshore Rocky Habitats. PloS ONE, 5(11): e13845 doi:10.1371/journal.pone.0013845. Joseph, L. (2005) Review of the Beche de mer (Sea Cucumber) Fishery in the Maldives. Technical Report 79, Food and Agriculture Organization of the United Nations, Madras, India. Juinio-Menez, M.A., Macawaris, N.N.D., & Bangi, H.G.P. (1998) Community-based sea urchin (Tripneustes gratilla) grow-out culture as a resource management tool. Can. Spec Publ. Fish. Aquat. Sci., 125: 393-399. Lovelock CE, Ellison J (2007) Vulnerability of mangroves and tidal wetlands of the Great Barrier Reef to climate change. In: Climate Change and the Great Barrier Reef: A Vulnerability Assessment (eds Johnson J, Marshall P), pp. 237–269. Great Barrier Reef Marine Park Authority and Australian Greenhouse Office, Townsville. Kaunda- Arara, B., Rose, G.A. (2004) Effects of marine reef National Parks on fishery CPUE in coastal Kenya. Biological Conservation, 118(1): 1-13. Kenya Wildlife Service. (2014) Watamu Marine National Reserve. Available at: http://cca.kws.go.ke/Watamu.html[Accessed 23 April 2014] Khamala, C.P.M. Ecology of Echinometra mathaei (Echinoidea: Echinodermata) at Diani Beach, Kenya. Marine Biology 11: 167-172 Mah, C.L. (2006) A new species of Xyloplax (Echinodermata: Asteroidea: Concentricycloidea) from the northeast Pacific: comparative morphology and a reassessment of phylogeny. Invertebrate Biology, 125(2): 136–153. Micael, J., Alves, M.J., Costa, A.C. & Jones, M.B. (2009) Exploitation and conservation of Echinoderms. Oceanography and Marine Biology: An Annual Review, 47: 191-208. Nash, W. & Ramofafia, C. (2006) Recent developments with the sea cucumber fishery in Solomon Islands. SPC Beche-de-mer Information Bulletin, 23: 3–4. Nishihira, M., Sato, Y., Arakaki, Y., Tsuchiya, M. (1991) Ecological distribution and habitat preference of four types of the sea urchin Echinometra mathaei on the Okinawan coral reefs: Biology of Echinodermata. Balkema Press, Rotterdam p.91-104. Oak, T., Scheibling, R.E. (2006) Tidal activity pattern and feeding behaviour of ophuiroid Ophiocoma scolopendrina on a Kenya reef flat. Journal of International Society of Reef Studies 25(2): 213-222. 37 Oigman-Pszczol, S., Figueiredo, M.A. de O., & Creed, J.C. (2004) Distribution of Benthic Communities on the Tropical Rocky Subtidal of Armacao dos Buzios, Southeastern Brazil. Marine Ecology, 25(3): 173-190. Orr J.C., Fabry V.J., Aumont O., Bopp L., Doney S.C., Feely R.A., Gnanadesikan A., Gruber N., Ishida A., Joos F., Key R.M., Lindsay K., Maier-Reimer E., Matear R., Monfray P., Mouchet A., Najjar R.G., Plattner G.K., Rodgers K.B., Sabine C.L., Sarmiento J.L., Schlitzer R., Slater R.D., Totterdell I.J., Weirig M.F., Yamanaka Y. & Yool A. (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature, 437:681–686. Tews, J., Brose, U., Grimm, V., Tielborger, K., Wichmann, M.C., Schwager, M., Jeltsch, F. (2004) Animal species diversity driven by heterogeneity/diversity: the importance of keystone structures. Journal of Biogeography, 31(1): 79-92 Totterdell I.J., Weirig M.F., Yamanaka Y. & Yool A. (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature, 437:681–686. Paine, R.T. (1966) Food web complexity and species diversity. The American Naturalist, 100: 65-75. Pawson, D.L. (2007) Phylum echinodermata. In: Linnaeus Tercentenary: Progress in Invertebrate Taxonomy, volume 1668 of Zootaxa (eds Z.Q. Zhang and W. Shear). Magnolia Press, Auckland, New Zealand. Przeslawski, R., Ahyong, S., Byrne, M., Worheides, G., Hutchings, G. (2008) Beyond corals and fish: the effects of climate change on noncoral benthic invertebrates of tropical reefs. Global Change Biology, 14: 2773-2795. Sammarco, P. W. (1980) Diadema and its relationship to coral spat mortality: grazing, competition, and biological disturbance. J. Exp. Mar. Biol. Ecol, 45: 245-272. Sammarco, P.W. (1982) Echinoid grazing as a structuring force in coral communities: whole reef manipulations. J. Exp. Mar. Biol. Ecol, 61: 31-55. Samoilys, M.A. (1988) Abundance and species richness of coral reef fish on the Kenyan coast: the effects of protective management and fishing. Proceedings of the 6th International Coral Reef Symposium, 2: 261-266 Sluko, R., Cowburn, B. & Jackson, C. (2012) The Impact of Watamu Marine National Park on Marine Biodiversity & Habitats. A Rocha Kenya Occasional Research Report # 24 Solomon S, Qin D, Manning M et al. (2007) Climate Change 2007: The Physical Science Basis. Contributions of Working Group I to the 4th Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge. 38 Uthicke, S. (1999) Sediment bioturbation and impact of feeding activity of Holothuria (Halodeima) atra and Stichopus chloronotus, two sediment feeding holothurians, at Lizard Island, Great Barrier Reef. Bulletin of Marine Science, 64: 129–141. Uthicke, S. (2001) Nutrient regeneration by abundant coral reef holothurians. Journal of Experimental Marine Biology and Ecology, 265: 153–170. Wabnitz, C., Taylor, M., Green, E., Raznak, T. (2003) From Ocean to Aquarium. Cambridge, UK.: UNEP- World Conservation Monitoring Centre. Zito F., Costa C., Sciarrino S., Cavalcante C., Poma V., & Matranga V. (2005) Cell adhesion and communication: a lesson from echinoderm embryos for the exploitation of new therapeutic tools. Prog Mol Subcell Biol, 39: 7–44. The following references were used to identify the echinoderms at the study sites: Humphreys, W.F. (1981) The Echinoderms of Kenya’s Marine Parks and adjacent regions. Documentation Zoologique N°19, Perth. Richmond, M.D. (2011) A Field Guide to the Sea shores of Eastern Africa and the Western Indian Ocean Islands Third Edition. Sida, WIOMSA. Samyn, Y. (2003) Shallow-Water Holothuroidea (Echinodermata) from Kenya and Pemba Island, Tanzania. Royal Museum for Central Africa, Tervuren Studies in Afrotropical Zoology Vol. 292 39 7. Appendix 7.1. Study site Locations Figure 14. A habitat map of the Watamu Marine Reserve. The rocky platforms used as study sites in the investigation are circled in red and the names of the rock platforms that were given them during the investigation are written beside each study site. The Map was provided by A Rocha Kenya. The Hemmingways Rock 1 and Turtle Bay Rock 2 study sites were connected however, due to the fact that most of the time the platforms appear separated as the connected rock part of the platform is underwater, the rock platform was considered as two study sites. 40 7.2. Assumptions of general linear model for Echinoderm species richness Figure 15. The bar chart of the standardized residuals of the number of echinoderm species. Figure 16. The normal Q-Q plot of the standardized residuals for the number of echinoderm species 41 Figure 17. A scatterplot of the predicted values and standardized residuals of the number of echinoderm species. There were no outliers in the echinoderm species richness data as determined by there being no cases with standardized residuals greater than +/-3 standard deviations. 7.3 Assumptions of general linear model for Echinoderm abundance Figure 18. A bar chart of the standardized residuals of the Echinoderm total organism count. 42 Figure 19. A bar chart of the standardized residuals of the total echinoderm organism count that had been transformed using square-root. Figure 20. The Normal Q-Q plot of the standardized residuals of the total echinoderm organism count transformed through square-root. 43 Figure 21. The scatter-plot of the predicted values and standardized residuals of the total echinoderm organism count that had been transformed through square-root. 7.4. Assumptions of the Logistic regression analyses Table 2. The results of the logistic analysis for the species Ophiocoma scolopendrina. The interaction terms between the seagrass cover percentages and their natural logs and the rockpool number and their natural logs are not statistically significant indicating that the original independent variables are linearly related to the logit of the dependent variable thereby meeting this assumption of logistic regression analysis. Variables in the Equation B Mode_rockpool_depth Floor_substratum Arcsine_seagrass_cover_percentages Step 1 a Rockpool_number LnRockpool_number by S.E. Wald df Sig. Exp(B) .155 .324 .230 1 .632 1.168 -.234 .270 .751 1 .386 .791 -1.119 1.027 1.186 1 .276 .327 -.279 .292 .913 1 .339 .757 .065 .071 .839 1 .360 1.067 .339 2.101 .026 1 .872 1.403 1.335 1.985 .452 1 .501 3.800 Rockpool_number Arcsine_seagrass_cover_percentages by LnArcs_living_seagrass_proportions Constant a. Variable(s) entered on step 1: Mode_rockpool_depth, Floor_substratum, Arcsine_seagrass_cover_percentages, Rockpool_number, LnRockpool_number * Rockpool_number , Arcsine_seagrass_cover_percentages * LnArcs_living_seagrass_proportions . 44 Table 3. The results of the logistic analysis species Amphiura dejectoides. The interaction terms between the seagrass cover percentages and their natural logs and the rockpool number and their natural logs are not statistically significant indicating that the original independent variables are linearly related to the logit of the dependent variable thereby meeting this assumption of logistic regression analysis. Variables in the Equation B 1 a Wald df Sig. Exp(B) Rockpool_number -.669 .622 1.156 1 .282 .512 Floor_substratum -.266 .604 .195 1 .659 .766 .743 .586 1.611 1 .204 2.103 -1.053 4.164 .064 1 .800 .349 -15.640 12.047 1.685 1 .194 .000 .193 .152 1.602 1 .206 1.212 -6.385 4.565 1.957 1 .162 .002 Mode_rockpool_depth Step S.E. Arcsine_seagrass_cover_percentages Arcsine_seagrass_cover_percentages by LnArcs_living_seagrass_proportions LnRockpool_number by Rockpool_number Constant a. Variable(s) entered on step 1: Rockpool_number, Floor_substratum, Mode_rockpool_depth, Arcsine_seagrass_cover_percentages, Arcsine_seagrass_cover_percentages * LnArcs_living_seagrass_proportions , LnRockpool_number * Rockpool_number . Table 4. The results of the logistic for the species Echinometra mathaei. The interaction terms between the seagrass cover percentages and their natural logs and the rockpool number and their natural logs are not statistically significant indicating that the original independent variables are linearly related to the logit of the dependent variable thereby meeting this assumption of logistic regression analysis. Variables in the Equation B S.E. Wald df Sig. Rockpool_number 1.548 1.403 1.218 1 .270 4.702 Floor_substratum -.640 .377 2.875 1 .090 .527 .279 .449 .386 1 .534 1.322 -1.898 1.409 1.815 1 .178 .150 1.589 2.895 .301 1 .583 4.899 -.495 .407 1.475 1 .225 .610 -3.091 4.811 .413 1 .521 .045 Mode_rockpool_depth Step 1 a Arcsine_seagrass_cover_percentages Arcsine_seagrass_cover_percentages by Exp(B) LnArcs_living_seagrass_proportions LnRockpool_number by Rockpool_number Constant a. Variable(s) entered on step 1: Rockpool_number, Floor_substratum, Mode_rockpool_depth, Arcsine_seagrass_cover_percentages, Arcsine_seagrass_cover_percentages * LnArcs_living_seagrass_proportions , LnRockpool_number * Rockpool_number . 45 Table 5. The results of the logistic analysis testing whether the continuous independent variables were linearly related to the logit of the dependent variable for the species Echinothrix diadema. The interaction terms between the seagrass cover percentages and their natural logs and the rockpool number and their natural logs are not statistically significant indicating that the original independent variables are linearly related to the logit of the dependent variable thereby meeting this assumption of logistic regression analysis. Variables in the Equation B S.E. Rockpool_number .659 1.506 Floor_substratum -.045 df Sig. .191 1 .662 1.932 .435 .011 1 .917 .956 .651 .525 1.537 1 .215 1.917 -2.979 1.892 2.479 1 .115 .051 3.357 3.434 .956 1 .328 28.697 -.231 .439 .278 1 .598 .793 -2.348 5.280 .198 1 .657 .096 Mode_rockpool_depth Step 1 a Arcsine_seagrass_cover_percentages Arcsine_seagrass_cover_percentages by Wald Exp(B) Ln_seagrass_cover_percentages Ln_rockpool_number by Rockpool_number Constant a. Variable(s) entered on step 1: Rockpool_number, Floor_substratum, Mode_rockpool_depth, Arcsine_seagrass_cover_percentages, Arcsine_seagrass_cover_percentages * Ln_seagrass_cover_percentages , Ln_rockpool_number * Rockpool_number . Table 6. The results of the logistic analysis testing whether the continuous independent variables were linearly related to the logit of the dependent variable for the species Tripneustes gratilla. The interaction terms between the seagrass cover percentages and their natural logs and the rockpool number and their natural logs are not statistically significant indicating that the original independent variables are linearly related to the logit of the dependent variable thereby meeting this assumption of logistic regression analysis . Variables in the Equation B S.E. Wald df Sig. 14.670 12.728 1.328 1 .249 2349598.565 -.202 .624 .105 1 .746 .817 .635 .788 .650 1 .420 1.888 Arcsine_seagrass_cover_percentages -2.166 2.944 .541 1 .462 .115 Arcsine_seagrass_cover_percentages by -1.620 6.820 .056 1 .812 .198 -4.076 3.492 1.363 1 .243 .017 -57.034 49.235 1.342 1 .247 .000 Rockpool_number Floor_substratum Mode_rockpool_depth Step 1 a Exp(B) Ln_seagrass_cover_percentages Ln_rockpool_number by Rockpool_number Constant a. Variable(s) entered on step 1: Rockpool_number, Floor_substratum, Mode_rockpool_depth, Arcsine_seagrass_cover_percentages, Arcsine_seagrass_cover_percentages * Ln_seagrass_cover_percentages , Ln_rockpool_number * Rockpool_number . 46 7.5 Results of Logistic regression analysis Table 7. Logistic regression analysis of effect of predictor variables on the presence or absence of Ophiocoma scolopendrina. Variables in the Equation B S.E. Wal d d f Sig. Exp(B) 95% C.I.for EXP(B) Lowe Upper r Rockpool_number .020 .024 Floor_substratum Floor_substratum(1) Floor_substratum(2) Floor_substratum(3) Floor_substratum(4) p1 .957 44708.88 Mode_rockpool_depth(3) Mode_rockpool_depth(4) Arcsine_seagrass_cover_percenta 3.61 4 .461 .000 1 0 1.069 1.130 1.020 .973 1.069 1.00 2.604 .000 . 2.912 .318 26.68 0 .895 1 .344 7 .502 1.178 .182 1 .670 1.653 1.502 1.163 1.66 1 .197 4.489 .459 7 16.63 5.73 3 43.85 2 .125 9 19.84 19580.83 8 .000 1 .999 416923473.06 4 20.78 19580.83 4 .000 1 .999 4 20.02 19580.83 1062302748.1 .000 1 .999 498330083.30 4 -.666 .735 .821 1 .365 .514 - 19580.83 .000 1 .999 .000 0 a. Variable(s) entered on step 1: Rockpool_number, Floor_substratum, Mode_rockpool_depth, 47 .000 . .000 . .122 2.170 1 4 Arcsine_seagrass_cover_percentages. . 87 7 22.16 .000 2 ges Constant .164 5 Mode_rockpool_depth Mode_rockpool_depth(2) .407 2 Ste a .687 1 Table 8. Logistic regression analysis of effect of predictor variables on the presense or absence of Amphiura dejectoides. Variables in the Equation B S.E. Wald d Sig. Exp(B) 95% C.I.for f EXP(B) Lowe Uppe r Rockpool_number .081 .039 Floor_substratum(2) Floor_substratum(3) Floor_substratum(4) 17.66 45879.27 9 7 18.06 12778.04 9 2 -1.027 14425.98 p1 18.46 12778.04 9 2 18059.77 4 2 18.01 18059.77 5 2 17.53 18059.77 3 2 Arcsine_seagrass_cover_percentag -1.494 Mode_rockpool_depth(4) .000 1 1.00 47164135.795 . 0 .000 1 .999 70338015.399 .000 . .000 1 1.00 .358 .000 . .999 104955586.79 .000 . 0 .000 1 6 .532 .000 1 .999 21728931.856 .000 . .000 1 .999 66635253.897 .000 . .000 1 .999 41170038.359 .000 . 1.779 .706 1 .401 .224 .007 7.331 - 22123.13 .000 1 .999 .000 38.87 7 es Constant .000 0 16.89 Mode_rockpool_depth(3) .997 2.20 3 Mode_rockpool_depth Mode_rockpool_depth(2) 1.084 1.004 1.171 .170 4 9 Ste a .039 9 Floor_substratum Floor_substratum(1) 4.26 1 r 2 a. Variable(s) entered on step 1: Rockpool_number, Floor_substratum, Mode_rockpool_depth, Arcsine_seagrass_cover_percentages. 48 Table 9. Logistic regression analysis of effect of predictor variables on the presense or absence of Echinometra mathaei in the transects. Variables in the Equation B S.E. Wald df Sig. Exp(B) 95% C.I.for EXP(B) Lower Rockpool_number -.067 .042 2.583 1 .108 4.701 4 .319 Floor_substratum Floor_substratum(1) Step 1 a - 40192.970 Upper .935 .862 1.015 .000 1 1.000 .000 .000 . 21.714 Floor_substratum(2) -.610 .960 .404 1 .525 .543 .083 3.566 Floor_substratum(3) .652 .964 .457 1 .499 1.919 .290 12.695 Floor_substratum(4) -.129 .975 .017 1 .895 .879 .130 5.940 2.467 3 .481 Mode_rockpool_depth Mode_rockpool_depth(2) -1.250 1.095 1.304 1 .253 .286 .033 2.449 Mode_rockpool_depth(3) -1.385 1.127 1.511 1 .219 .250 .027 2.279 Mode_rockpool_depth(4) -.645 1.191 .293 1 .588 .525 .051 5.420 Arcsine_seagrass_cover_percentages -.823 .923 .796 1 .372 .439 .072 2.679 .511 1.218 .176 1 .675 1.667 Constant a. Variable(s) entered on step 1: Rockpool_number, Floor_substratum, Mode_rockpool_depth, Arcsine_seagrass_cover_percentages. 49 Table 10. Logistic regression analysis of effect of predictor variables on the presense or absence of Echinothrix diadema in the transects. Variables in the Equation B S.E. Wald df Sig. Exp(B) 95% C.I.for EXP(B) Lower Upper Rockpool_number -.042 .051 .959 .867 1.060 1 1.000 .000 .000 . 1.077 3.189 1 .074 .146 .018 1.206 Floor_substratum Floor_substratum(1) Step 1 a - 40192.970 .672 1 .412 4.735 4 .316 .000 21.180 Floor_substratum(2) -1.923 Floor_substratum(3) -.523 1.028 .259 1 .611 .593 .079 4.445 Floor_substratum(4) -.441 .999 .195 1 .659 .643 .091 4.554 3.322 3 .345 Mode_rockpool_depth Mode_rockpool_depth(2) -.753 1.291 .340 1 .560 .471 .038 5.909 Mode_rockpool_depth(3) -.711 1.323 .289 1 .591 .491 .037 6.565 Mode_rockpool_depth(4) .534 1.364 .153 1 .696 1.705 1.307 1.914 1 .167 .164 1.371 1 .987 .977 Arcsine_seagrass_cover_percentages Constant -1.808 -.023 .000 a. Variable(s) entered on step 1: Rockpool_number, Floor_substratum, Mode_rockpool_depth, Arcsine_seagrass_cover_percentages. 50 .118 24.715 .013 2.124 Table 11. Logistic regression analysis of effect of predictor variables on the presense or absence of Tripneustes gratilla. Variables in the Equation B S.E. Wald d Sig. Exp(B) 95% C.I.for f EXP(B) Lowe Uppe r Rockpool_number -.072 .058 Floor_substratum(2) Floor_substratum(3) Floor_substratum(4) .218 .931 .830 1.043 6 Floor_substratum Floor_substratum(1) 1.51 1 r 17.75 46297.65 1 5 18.67 13002.17 8 9 18.99 13002.17 7 9 17.86 13002.17 7 9 .803 4 .938 .000 1 1.00 51167641.599 .000 . .000 . .000 . .000 . .000 . .000 . .000 . 0 .000 1 .999 129284052.52 0 .000 1 .999 177881599.02 7 .000 1 .999 57465098.780 .478 3 .924 .000 1 .999 138090004.97 Ste p1 a Mode_rockpool_depth 18.74 18945.74 3 0 18.60 18945.74 4 0 19.27 18945.74 2 0 Arcsine_seagrass_cover_percentag -1.291 1.636 .622 1 .430 .275 - 22978.20 .000 1 .999 .000 38.95 7 Mode_rockpool_depth(2) Mode_rockpool_depth(3) Mode_rockpool_depth(4) 5 .000 1 .999 120151846.43 8 .000 1 .999 234351918.85 4 es Constant 4 a. Variable(s) entered on step 1: Rockpool_number, Floor_substratum, Mode_rockpool_depth, Arcsine_seagrass_cover_percentages. 51 .011 6.796 7.6 Monotopic assumption of Spearman’s rank correlation between Echinothrix diadema and Echinometra mathaei Visual inspection of the scatter-plot (figure 23) of the paired abundance data of these two species did not appear to be monotopic and so the data was transformed with the natural log in order to produce a monotopic scatter-plot (figure 24), thus meeting this assumption. Figure 23. The scatterplot comparing the paired abundance data of Echinometra mathaei and Echinothrix diadema. The relationship does not appear to be strictly monotopic; as one variable increases, the other one does not always increase. 52 Figure 24. The scatterplot comparing the natural log transformed, paired abundance data of Echinometra mathaei and Echinothrix diadema. Here the relationship loosely appears to be monotopic but not linearly monotopic. 53