CHEM 135 General Chemistry for Engineers

CHEM 135 General Chemistry for Engineers - Fall 2012
Dr. Andrei Vedernikov
Instructor Contact Information:
Office: CHM 2353
Everyone is welcome to stop by my office. The office is in the 3rd (new) wing of the Chemistry Building.
To get to this area you need to take the new large elevator on the ground floor or the 1st floor of the
Building at the intersection of the 2nd and 3rd wings.
E-mail: avederni@umd.edu
Teaching Assistant Information:
Navadeep Boruah
Teaching Assistant e-mail address: navadeepboruah@gmail.com
Required Materials:
1) Text by N.J. Tro. Chemistry. A Molecular Approach , 2 nd Ed., Prentice Hall, 2011; customized for the
University of Maryland, along with
Registration Code for Mastering Chemistry online homework system ISBN 978-0-5-5873604-0. It is cost-wise to buy this package at the University Bookstore.
2)
Mastering Chemistry
(online home work system)
Registration Code
is included in the package above; if you buy text w/o MC Registration Code, the latter can be bought separately: ISBN 978-0-3-2169533-8.
3) Clicker Response Card RF-LCD by Response Innovations (to earn points for clicker questions in class); ask at the counter at the University Bookstore.
Lecture Time and Place:
MWF, 2:00-2:50 pm; CHM 1407
Office Hours: MWF, 3:00-4:00 pm; Tu, 11:00 am – 1 pm;Th, 12:30 – 1:30 pm.
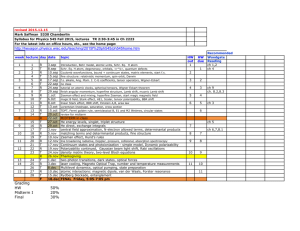
Grading in the course
Clicker Questions
Assignment
2 points
Total points
Assignments
10 questions counted 20 points
Exams
Final Exam
TOTAL
100 points
200 points
Grades, percent of the total number of points (points total): counted
3 exams
1 exam
300 points
200 points
620 points
A , ≥ 85% ( ≥ 524 pts); B , ≥ 75% ( ≥ 462 pts); C , ≥ 65% ( ≥ 400 pts); D , ≥ 55% ( ≥ 338 pts); F , <55%
I. Clicker Questions, Homeworks and Exams
Clickers in the Classroom
: We will be using the Personal Response System devices (clickers) in class. Clickers can be purchased at the University bookstore by asking at the counter on the lower level.
Once you purchase a new clicker or replace a lost one you must register it
with the university. An unregistered clicker will be useless in class
. Go to http://www.clickers.umd.edu
(a picture of the
Response Card RF-LCD is on top on the page) and follow the Students link. This website will give you information on purchasing your clicker, registering your clicker, using your clicker and what to do if your clicker is lost/stolen. Our classroom channel to be used with clickers is 45 (accessible only when we use clickers in class).
Alternatively, you can use a Web Accessible Mobile Device
(iPhone/iPod/iPad, Blackberry,
laptop, etc)
. In such a case you will need to buy a license
from
ResponseWare
and learn how to use your device instead of a clicker (see instructions at http://clickers.umd.edu/students/index_students.html
). Our classroom ResponseWare session ID is
AV2012
(the session is open only when we use clickers in class).
There will be 20 clicker questions over the course of the semester, beginning
Sep. 10
, which you will be required to answer. These questions will be on the material recently covered in the course, which is a way to make sure you are keeping up with the material. Clicker questions may be given at any time during a class. Each clicker question is worth 2 points toward your final grade. Your 10 lowest clicker scores will be dropped when figuring out your final grade in the course. You will be able to keep track of your clicker points on the
Blackboard
. Clicker questions make up will NOT be possible.
Homeworks
:
We will be using the
Mastering Chemistry
on-line home work system. You need a
Mastering Chemistry Registration Code to gain an access to the website and complete assignments.
The Mastering Chemistry registration code is included along with your text in a package sold in the
University bookstore. If you purchased your text w/o the Registration Code you will need to purchase
Mastering Chemistry Registration Code separately at the University Bookstore or by going to http://www.masteringchemistry.com/site/register/new-students.html
.
All students will need to set up their
Mastering Chemistry
account before you can get started.
Our 2 pm class Course ID with Mastering Chemistry is AV2012 . Instructions for registering with
Mastering Chemistry and logging into the system and troubleshooting can be found on the Blackboard .
There will be thirteen homework assignments this term that you must submit. One more assignment
“Introduction to Mastering Chemistry”
does not give you points but is highly recommended to be completed before you begin working on your first actual homework. Your three lowest homework grades will be dropped when figuring out your final grade in the course. The number of questions in each assignment may vary. The total of 10 points can be earned for each assignment. You will have three attempts for each question. For not using hints when answering a particular question you are rewarded with a bonus up to 30%. Typically, you will be given one week to complete each assignment.
The places to keep track of when assignments are due are the syllabus and the assignment page on our section Mastering Chemistry web-site. Most of the assignments will be released at 3:00 PM on
Mondays and, except HW13, are due at 2:00 PM the next Monday. Note that no HW is due on Sep. 3,
Nov. 5 and Dec. 10.
Exams:
The 50 min exams will be on October 3, November 2 and December 7. The final 2 hour exam is December 17 (1:30 am -3:30 pm).
All the exams are cumulative with emphasis placed on the new material.
Non-programmable
calculators will be allowed during the exams. A scientific calculator is recommended.
No electronic devices other than your calculator will be permitted during any exam.
Each student is required to keep his/her UM photo ID on the desk during the exam.
If you know you will have a conflict with the dates of the exam, such as religious observances, you must be in touch with your instructor by the end of schedule adjustment (September 12).
If you are ill on the day of the exam, you must contact your professor before the exam to make alternative arrangements. An e-mail or phone message
PRIOR
to the exam time would suffice.
Make up exams will be given only with a valid documented University excuse.
University-Excused absences
. It is the policy of the University to excuse the absences of students if the absence results from the following causes: illness of the student, or illness of a dependent as defined by Board of Regents policy on family and medical leave; religious observance where the nature of the observance prevents the student from being present during the class period; participation in university activities at the request of University authorities; and compelling circumstance beyond the student's control. Students requesting an exam make-up due to an excused absence must apply in writing and furnish documentary support for their assertion that the absence resulted from one of these causes.
II. The Blackboard
We will be using the
Blackboard Academic Suite
in this course
( https://elms.umd.edu/webapps/portal/frameset.jsp
). To log in onto
Blackboard
use your directory ID
(same as your terpmail.umd.edu email username) and password. After logging in, Click on
CHEM 135
to enter the site.
Resources on Blackboard :
The syllabus and illustrations to lectures
Instructions for registering with Mastering Chemistry
Some
old exams
and answer keys will be posted there. These exams can be used to prepare for your hourly and final exams.
The
My Grades
feature allows you to monitor your progress in the class. Be sure to check that your grades are accurately recorded. It is your responsibility to notify your instructor of any discrepancies in a timely manner.
III. Course Policies
Academic Integrity:
The University of Maryland, College Park has a nationally recognized Code of
Academic Integrity, administered by the Student Honor Council. This Code sets standards for academic integrity at Maryland for all undergraduate and graduate students. As a student you are responsible for upholding these standards for this course. It is very important for you to be aware of the consequences of cheating, fabrication, facilitation, and plagiarism. For more information on the Code of Academic
Integrity or the Student Honor Council, please visit: http://thestamp.umd.edu/gh/academics/academic_integrity
Students are responsible for knowing, understanding and behaving according to the content of the Code.
There will be zero tolerance for any violations.
Make-Up Exams:
Only university-excused absences are allowed. Proper documentation is expected.
Your instructor must be contacted within 24 hours of the missed exam. Failure to meet these three requirements results in a ZERO for the missed exam
.
Learning or other Disability
:
If you have a disability, please make an appointment to discuss available accommodations to maximize your learning experience in this course.
Learning disabilities must be documented by Disability Support Services prior to receiving accommodations
.
Exam Re-Grades:
All re-grades must be turned in to the TA the same day the exams are handed back and at no other time. Re-grades will be returned to the students within one week. Note, however, that the entire exam will be re-graded and this may result in a lower grade. Steps to take for re-grade:
1.
Assess why you feel you were unfairly graded
2.
On a separate piece of paper write an explanation for why you feel a certain problem should be regraded. Write clearly and be succinct. DO NOT WRITE ON YOUR EXAM.
3.
Staple your explanation to the front of your exam.
4.
Turn in re-grades to the Discussion TA.
Withdraw:
The last day for schedule adjustments is September 12 th
November 7 th .
. The last day to withdraw is
IV. How to Be Successful in the Class
Attendance is strongly suggested for lectures.
It is in your best interest to obtain lecture notes and supplementary materials for missed classes. You are responsible for: i) material included in our Lecture schedule
(see the list of Chapters covered either in full or in part on the last two pages of our Syllabus), ii) any additional material covered in class and iii) announcements made in class whether or not you are in attendance.
Questions are welcome during lecture. I strongly recommend that you write down your questions that you had no time to ask in class and ask them when you have a discussion session or visit me during my office hours. Please be respectful of others and turn your cell phones off.
The problems within and at the end of the chapters are critical to your success in the course.
The old saying is ‘practice makes perfect.’ Do not ever stop at the point when you just
“understand” the material. You are expected to develop skills at solving course-related problems. Practice at solving problems as much as you can. Remember, you will have only few minutes (3-5 min) per problem/question during any of your exams. This time should be sufficient for you to solve a problem and double check your answer!
Attend your Discussion section every week.
Discussion sections will begin meeting on
Tuesday, September 4 . You are expected to work through the end-of-chapter and homework problems before your discussion meeting, so you can effectively discuss these problems with your classmates and the TA. After you take an hour exam, graded exam will be handed back and solutions will be given online on the Blackboard and/or during your discussion section.
Guided Study Sessions: get extra help if you need it.
You will have Guided Study Sessions held in the evenings twice a week by Robin Alonge ( ralonge@umd.edu
). In addition, there is a list of tutors for hire in room 2102 (chemistry advising office).
V. Online Course Evaluation
Your participation in the evaluation of courses through CourseEvalUM is a responsibility
you hold as a student member of our academic community. Your feedback is confidential and important to the improvement of teaching and learning at the University. CourseEvalUM will be open for you to complete your evaluations for Fall 2012 courses from Tuesday, November 27 through Wednesday, December 12.
Please go directly to the website ( www.courseevalum.umd.edu
) to complete your evaluations.
Tentative lecture and exam schedule:
Week Date/Lecture Topic
1
W Aug 29 / Introduction.
2
F Aug 31 /1
Sep. 3, 2PM
Ch. 1 Atoms/molecules/elements/compounds/mixtures.
HW 1 is released (Ch. 1, 2 material)
M Sep 3 /
W Sep 5 /2
No classes. Labor day
Ch. 2 Structure of the atom. Atomic number, mass number. Isotopes; atomic and molar mass.
F Sep 7 /3 Ch. 2 The periodic table of the elements: an overview.
3 Sep 10, 2PM
HW 1 is due (Ch. 1, 2 material)/HW 2 is released (Ch. 3.1-3.6 material)
M Sep 10 /4 Ch. 3 Ionic and covalent substances. Chemical formulas and nomenclature. Naming acids.
W Sep 12 /5
4
F Sep 14 /6
W
F
Sep 17, 2PM
Sep 19 /8
Sep 21 /9
5 Sep 24, 2PM
Ch. 7 The electromagnetic spectrum. Atomic spectra. Schrodinger equation.
Ch. 7 The hydrogen atom: atomic orbitals and quantum numbers
M Sep 17 /7 Ch. 8 Electron-spin quantum number m n
,
HW 3 is due (Ch. 7 material)/HW 4 is released (Ch. 8 material) l
, m l
.
HW 2 is due (Ch. 3.1-3.6 material)/HW 3 is released (Ch. 7 material) s
. Multi-electron atoms: orbital energies, electron configuration
Ch. 8 Periodic trends: atomic radii, effective nuclear charge and ionization energy.
Ch. 8 Electron configuration of ions. Paramagnetism. Ionic radii and their trends.
M Sep 24 /10 Ch. 9 Lewis structures. The octet rule. Ionic bonding: lattice energy.
W Sep 26 /11 Ch. 9 Electronegativity & bond polarity. Writing Lewis structures.
F Sep 28 /12
6 Oct 1, 2PM
Ch. 9 Formal charge. Resonance structures. Exceptions to the octet rule.
HW 4 is due (Ch. 8 material)/HW 5 is released (Ch. 9 material)
M Oct 1 /13 Ch. 9 Covalent bond length and energy.
W Oct 3 /
F Oct 5 / 14
7 Oct 8, 2PM
Midterm exam 1 / chapters 1, 2, 3.1-3.6, 7, 8
Ch. 10 VSEPR. Valence electron-group arrangements & molecular shapes.
HW 5 is due (Ch. 9 material)/HW 6 is released (Ch. 10 material)
M Oct 8 /15 Ch. 10 Using VSEPR theory to determine molecular shape & molecular polarity.
W Oct 10 /16 Ch. 10 Covalent bonding: valence bond theory. Atomic orbital hybridization. - & -bonds.
F Oct 12 /17 Ch. 3 Empirical formula and composition of a compound. Mass percent. Molecular formula.
8 Oct 15, 2PM
HW 6 is due (Ch. 10 material)/HW 7 is released (Ch. 3.7-3.10 material)
M Oct 15 /18 Ch. 3 Writing chemical equations & balancing them.
W Oct 17 /19 Ch. 4 Stoichiometry Calculations. Concentration. Percent yield. Limiting Reactant.
9
F Oct 19 /20
Oct 22, 2PM
Ch. 4 Compounds in aqueous solutions. Strong & weak electrolytes; non-electrolytes.
HW 7 is due (Ch. 3.7-3.10 material)/HW 8 is released (Ch. 4 material)
M Oct 22 /21 Ch. 4 Solubility. Precipitation reactions. Molecular and ionic equations.
W Oct 24 /22
F Oct 26 /23
10 Oct 29, 2PM
Ch. 4 Acid-base reactions. Gas evolution reactions.
Ch. 4 Redox Reactions. Oxidation states.
HW 8 is due (Ch. 4 material)/HW 9 is released (Ch. 5, 6 material)
M Oct 29 /24 Ch. 5 The gas laws. Stoichiometry calculations with gases.
W Oct 31 /25
W Nov 7 /27
F Nov 9 /28
Ch. 6 Calorimetry, thermochemical equations. Standard enthalpy of a reaction. Hess’s law.
F Nov 2
11 Nov 5, 2PM
No HW is due
Midterm exam 2 / chapters 3.7-3.10, 4, 9, 10
M Nov 5 /26 Ch. 11 Intermolecular forces.
Ch. 11 Liquids and solids.
Ch. 12 Solutions: an overview.
12 Nov 12, 2PM
HW 9 is due (Ch. 5, 6 material)/HW 10 is released (Ch. 11, 12, 13 material)
M Nov 12/29 Ch. 13 The rate of a chemical reaction. Rate laws. Temperature and reaction kinetics.
W Nov 14/30 Ch. 14 Chemical equilibrium. The equilibrium constant & the reaction quotient.
F Nov 16/31
13 Nov 19, 2PM
HW 10 is due (Ch. 11, 12, 13 material)/HW 11 is released (Ch. 14 material)
M Nov 19/32 Ch. 15 Acid-base definitions. Acidity constant K
W
F
Nov 21/33
Nov 23
14 Nov 26, 2PM
Ch. 14 Calculating equilibrium concentrations. Le Châtelier’s principle. a
; the pH scale.
Ch. 15 Solving problems involving acid/base equilibria.
Thanksgiving break
HW 11 is due (Ch. 14 material)/HW 12 is released (Ch. 15, 16 material)
M Nov 26/34 Ch. 16 Ionic equilibria in aqueous solutions: solubility/precipitation.
W Nov 28/35 Ch. 18 Voltaic cells. Batteries and fuel cells.
F Nov 30/36
15 Dec 3, 2PM
Ch. 18 Electrolysis and corrosion.
HW 12 is due (Ch. 15, 16 material)/HW 13 is released (Ch. 18, 20 material)
M Dec 3 / 37 Ch. 20 Classification and nomenclature of organic compounds. Functional groups. Isomerism.
W Dec 5 / 38 Ch. 20 An overview of organic reactions. Part 1.
F Dec 7 Midterm exam 3 / chapters 5, 6, 11-16
16 Dec 10, 2 PM
No HW is due
M Dec 10 / 39 Ch. 20 An overview of organic reactions. Part 2.
W Dec 12, 2PM
HW 13 is due (Ch. 18, 20 material)
17
M Dec. 17
Final exam at 1:30-3:30 pm / all above