Fundamental ideas in chemistry questions
advertisement

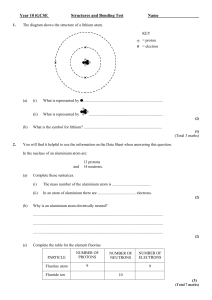
## The table gives some information about a family of molecules in crude oil. (a) NUMBER OF CARBON ATOMS IN MOLECULE MASS OF MOLECULE (atomic units) 1 16 2 30 4 58 Show information from the table in the most appropriate way on the grid. (3) (b) What is the mass of a molecule with three carbon atoms? ..................................................................................................................................... (1) (c) The other atoms in each molecule are all hydrogen atoms. What family of substances do all the molecules belong to? ..................................................................................................................................... (1) Page 1 of 34 (d) The mass of a carbon atom is 12 atomic units. The mass of a hydrogen atom is 1 atomic unit. So the molecule with one carbon atom has four hydrogen atoms. Its formula is CH4. Write down the formula: (i) of the molecule with two carbon atoms (ii) of a molecule from the same family with five carbon atoms ...................... ...................... (2) (Total 7 marks) Q2. Sodium reacts with water to produce hydrogen gas and a solution of sodium hydroxide. Complete the word equation for this reaction (do not use symbols or formulae). ............................. + ....................... ............................ + ........................... (Total 3 marks) Q3. The diagram shows part of the periodic table. Choose from the elements shown in the table: (a) one metal .................................................................................................................... (1) (b) a noble gas ................................................................................................................. (1) (c) a coloured gas ............................................................................................................ (1) (Total 3 marks) Page 2 of 34 Q4. The diagram shows the structure of a lithium atom. (a) (i) What is represented by ................................................................................. (ii) What is represented by ........................................................................... (2) (b) What is the symbol for lithium? ................................................................................ (1) (Total 3 marks) Q5. The formula for the compound hydrogen peroxide is H2O2. Write down everything that the formula tells you about each molecule of hydrogen peroxide. ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... (Total 4 marks) Page 3 of 34 Q6. Here is the word equation for a chemical reaction. magnesium + zinc oxide → magnesium oxide + zinc Write down everything that the word equation tells you about the reaction. ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... (Total 4 marks) Q7. Choose gases from this list to complete the word equations below. (a) carbon dioxide hydrogen oxygen sulphur dioxide nitrogen sodium + water → sodium hydroxide + ............................................. . (1) (b) magnesium + ............................................................→ magnesium oxide. (1) (Total 2 marks) Q8. (a) (i) Balance these chemical equations. H2 + O2 → H 2O (1) (ii) Al + O2 → Al 2O3 (1) (b) Briefly explain why an unbalanced chemical equation cannot fully describe a reaction. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (2) (Total 4 marks) Page 4 of 34 Q9. Here is the word equation for a chemical reaction. methane + oxygen → water + carbon dioxide Write down everything that the word equation tells you about the reaction. ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... (Total 4 marks) Q10. Choose words from this list to complete the sentences below. carbonate (a) chloride compound mixture oxide solution When two elements react, the new substance formed is called a .............................. . (1) (b) The white powder formed when zinc reacts with oxygen is called zinc .......................... . (1) (Total 2 marks) Q11. (a) Sulphur is a yellow element. It is a non-metal. (i) Complete the sentence. In an element, all the atoms ............................................................................. .......................................................................................................................... (1) (ii) Give two properties you would expect sulphur to have because it is a non-metal. 1. ...................................................................................................................... .......................................................................................................................... 2. ...................................................................................................................... .......................................................................................................................... (2) Page 5 of 34 (b) Use the names of metals from the box to complete the table. copper iron magnesium manganese zinc Use Name of metal for electric wiring in a house ....................................................... for manhole covers ....................................................... to galvanise iron ....................................................... (3) (c) Copper is used to make hot water pipes. Both iron and steel are cheaper. Suggest two reasons why copper is used rather than iron or steel. 1. ................................................................................................................................. ..................................................................................................................................... 2. ................................................................................................................................. ..................................................................................................................................... (2) (d) The drawing shows a container of a compound called sodium chloride. (i) Which other element has combined with sodium to form this compound? .......................................................................................................................... (1) (ii) For every atom of sodium, how many atoms of the other element have combined with it? .......................................................................................................................... (1) (Total 10 marks) Page 6 of 34 Q12. The diagram shows an atom. (a) On the diagram, write the names of structures A, B, C and D. (4) (b) To which Group of the periodic table does this atom belong? ..................................................................................................................................... Give one reason for your answer. ..................................................................................................................................... ..................................................................................................................................... (2) (c) Name the element which is made up of this type of atom. ..................................................................................................................................... (1) (Total 7 marks) Q13. Use the Periodic Table of Elements on the Data Sheet to help you to answer this question. (a) Describe, in as much detail as you can, the structure of a fluorine atom. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (3) Page 7 of 34 (b) Complete the diagram to show the electronic structure of a magnesium atom. (1) (Total 4 marks) Q14. (a) Helium is used to fill party balloons. Which two of the following are properties that make helium suitable for filling party balloons? Place a tick ( ) in the box against each suitable property. Coloured Exists as individual atoms Less dense than air Poor conductor of heat Very unreactive (2) Page 8 of 34 (b) The table shows the names of some gases. Use the correct formulae from the box to complete the table. The first one has been done for you. CH4 CO2 H2 Gas Oxygen HCl NH3 O2 Formula O2 Carbon dioxide Hydrogen chloride Ammonia (3) (Total 5 marks) Q15. The periodic table on the Data Sheet might help you to answer this question. Diagrams A – D show models of four different molecules. Complete the table to give the name and the formula of each of the molecules A – D. The first one has been done for you. Molecule Name Formula A Hydrogen H2 B C D (Total 6 marks) Page 9 of 34 Q16. One definition of an element is: “A substance that cannot be broken down into simpler substances by chemical methods” The table below shows some of the ‘substances’ which Antoine Lavoisier thought were elements. He divided the ‘substances’ into four groups. He published these groups in 1789. The modern names of some of the ‘substances’ are given in brackets. ACID-MAKING ELEMENTS sulphur phosphorus charcoal (carbon) GAS-LIKE ELEMENTS METALLIC ELEMENTS EARTHY ELEMENTS light cobalt mercury lime (calcium oxide) caloric (heat) copper nickel magnesia (magnesium oxide) oxygen gold azote (nitrogen) iron silver argilla (aluminium oxide) hydrogen lead tin silex (silicon dioxide) magnese tungsten platina (platinum) barytes (barium sulphate) zinc (a) Name one ‘substance’ in the list which is not a chemical element or compound. ..................................................................................................................................... (1) (b) (i) Name one substance in the list which is a compound. ............................................................................................................................ (1) (ii) Suggest why Lavoisier thought that this substance was an element. ............................................................................................................................ ............................................................................................................................ ............................................................................................................................ ............................................................................................................................ (1) (Total 3 marks) Page 10 of 34 Q17. The diagram represents an atom. Choose words from the list to label the diagram. electron ion neutron nucleus (Total 3 marks) ## Sando-K is a medicine. It is given to people whose bodies contain too little of a particular element. Sando-K is a mixture of two compounds. The formulae of the two compounds are given below. KHCO3 (a) KCl Use the Data Sheet to help you to name all the elements in these compounds. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (3) (b) Which metal do people given Sando-K need? ..................................................................................................................................... (1) (Total 4 marks) Page 11 of 34 Q19. (a) The list below gives six substances. • aluminium • beer • copper • milk • pure water • sodium chloride Put each substance in the correct column of the table. ELEMENTS COMPOUNDS MIXTURES (3) (b) Elements can be divided into two groups, metals and non-metals. The list below gives some properties of elements. • brittle • can be hammered into shape • dull • good conductors of electricity • poor conductors of electricity • shiny Put each property into the correct column. PROPERTIES OF METALS PROPERTIES OF NON-METALS (3) (Total 6 marks) Page 12 of 34 Q20. One step in the manufacture of lead is the reduction of lead oxide with carbon. Lead and carbon dioxide are the products of this reaction. (a) Write a word equation for this reaction. ..................................................................................................................................... (1) (b) What is meant by “reduction”? ..................................................................................................................................... (1) (Total 2 marks) Q21. There are millions of different substances that make up our world. All these substances are made from chemical elements. (a) What is an element? ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (1) (b) Many substances are compounds. What is a compound? ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (2) (Total 3 marks) Page 13 of 34 Q22. About 100 years ago a scientist called J. J. Thomson thought that an atom was a ball of positive charge with negative particles stuck inside. Today a different model is used. The diagram shows how an atom of carbon is represented by this model. (a) The negative particles (i) are called electrons. What is the name of the positive particles ? ........................................................................................................................... (1) (ii) What particle is represented by ●? ........................................................................................................................... (1) (iii) What is the central part of the atom called that contains both and ●? ........................................................................................................................... (1) (b) Use the model to explain why the six electrons are arranged as shown. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (2) (Total 5 marks) Page 14 of 34 Q23. In the flasks are the particles of four different gases. (Each circle represents an atom.) (a) Which diagram represents (i) oxygen, O2 .................................. (1) (ii) steam, H2O ................................. (1) (b) The gases in A and B are elements and the gases in C and D are compounds. Explain why. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (3) (Total 5 marks) Q24. The diagram shows the reaction of hydrogen molecules with oxygen molecules to form water molecules. (i) In the empty box draw one oxygen molecule. (1) Page 15 of 34 (ii) Why are hydrogen and oxygen called elements? ..................................................................................................................................... ..................................................................................................................................... (1) (iii) Why is water called a compound? ..................................................................................................................................... ..................................................................................................................................... (2) (Total 4 marks) Q25. To make crude oil more useful it is separated into different fractions. (a) Complete the gaps in the following sentences. Crude oil is separated into different fractions by a process called ............................ .................................. . Each fraction has a different ............................................... . (2) Page 16 of 34 (b) Each fraction is a mixture of compounds. Most of these compounds are hydrocarbons, made up of the elements hydrogen and carbon. (i) Explain the difference between a mixture and a compound. .......................................................................................................................... .......................................................................................................................... .......................................................................................................................... (2) (ii) Explain the difference between a compound and an element. .......................................................................................................................... .......................................................................................................................... .......................................................................................................................... (2) (Total 6 marks) Q26. The diagram shows a light bulb. (a) (i) An argon atom has the structure shown. Use the words in the box to label the particles in the atom. Each word should only be used once. electron neutron proton (2) Page 17 of 34 (ii) Argon is unreactive. Why? ..................................................................................................................... ..................................................................................................................... (1) (b) Oxygen would not be a suitable gas to use in a light bulb. Explain why. ................................................................................................................................ ................................................................................................................................ (2) (Total 5 marks) Q27. The diagrams show the electronic arrangement of the atoms of two elements. (i) Name the part of the atoms labelled X. .................................................................................................................................... (1) (ii) Why are these two elements in the same group of the Periodic Table? .................................................................................................................................... .................................................................................................................................... (1) (Total 2 marks) Page 18 of 34 Q28. (a) The diagram represents an atom of beryllium. Use words from the box to label the diagram. electron ion isotope molecule nucleus (2) (b) Use crosses (x) to complete the diagram to show the electronic structure of a magnesium atom. The atomic (proton) number of magnesium is 12. (2) (Total 4 marks) Q29. The diagram shows an electric light bulb. When electricity is passed through the tungsten filament it gets very hot and gives out light. Page 19 of 34 (a) What reaction would take place if the hot tungsten was surrounded by air? ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (1) (b) State why argon is used in the light bulb. Explain your answer in terms of the electronic structure of an argon atom. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (3) (Total 4 marks) Q30. The diagram shows an electric light bulb. When electricity is passed through the tungsten filament it gets very hot and gives out light. (a) What reaction would take place if the hot tungsten was surrounded by air? ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (1) Page 20 of 34 (b) State why argon is used in the light bulb. Explain your answer in terms of the electronic structure of an argon atom. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (3) (Total 4 marks) Page 21 of 34 M1. (a) • vertical axis appropriately scaled [i.e. using more than half the grid] • all three points correctly plotted* (to < ½ a square) • reasonably straight line drawn through points (to < half a square)* [*credit both these marks for bars correctly drawn since discontinuous variable] each • for 1 mark x [If points incorrectly plotted credit 1 mark for the best fit straight line or curve but not point-to-point] 3 (b) 44 (atomic units) for 1 mark (e.c.f. i.e. credit consistent with candidate’s graph) 1 (c) hydrocarbons / alkanes for 1 mark 1 (d) C2H6 C5H12 each for 1 mark [NB figures must be subscripted] 2 [7] M2. • correct reactants (i.e. sodium + water either way round) • correct products (i.e. sodium hydroxide + hydrogen, either way round) • arrow → / = [do not allow produce/makes or similar] [do not allow symbols or formulae] each for 1 mark [3] Page 22 of 34 M3. (a) sodium / magnesium / aluminium (Allow correct symbols) for 1 mark 1 (b) argon (Allow correct symbols) for 1 mark 1 (c) chlorine (Allow correct symbols) for 1 mark 1 [3] M4. (a) (i) neutron (name only) 2 (ii) (b) nucleus / protons and neutrons each for 1 mark (do not allow mass number) Li (correct cases of letters required) for 1 mark 1 [3] M5. contains oxygen atoms contains hydrogen atoms atoms are [chemically] bonded ratio of two hydrogen to two oxygen atoms each for 1 mark [4] ## correct use of ‘react’/‘reaction’/reactants’/combine (not mixed/added/join) correct use of ‘produce’/‘products’/gives/forms/makes/creates reactants correctly identified each for 1 mark products correctly identified (Reactants must be correctly identified for ‘react’ mark to be awarded. Similarly for products) (magnesium reacts with zinc oxide to produce magnesium oxide and zinc or similar, will gain all 4 marks) Oxidise or reduce given correctly can be credited both the marks for react and produce [4] Page 23 of 34 M7. (a) hydrogen for 1 mark 1 (b) oxygen for 1 mark 1 [2] M8. (a) (i) H2 + O2 → H2O *both circled correct for 1 mark 1 (ii) A1 + O2 → A12O3 all circled correct for 1 mark 1 (b) idea that: must end up with the same number of atoms as at the start any 2 each otherwise matter is shown to be lost/gained for 1 mark won’t show correct amount of each element/compound 2 [4] M9. idea of react/reactant/burn/combine idea of produce/product/formed/make (NOT a chemical symbol equation) methane + oxygen identified as reactants carbon dioxide + water identified as products each for 1 mark [4] M10. (a) compound 1 (b) oxide 1 [2] Page 24 of 34 M11. (a) (i) are identical / the same or have the same number of protons / the same proton number / electrons not similar 1 (ii) any two of low melting point low boiling point brittle (solid) do not credit just solid poor conductor of heat or heat insulator poor conductor of electricity or electrical insulator accept just poor / low conductor or just insulator once only dull surface or not shiny cannot be hammered / bent (into shape) or not malleable cannot be stretched (into shape) or not ductile does not make a clanging sound (when struck) or not sonorous acidic oxides low density 2 (b) copper 1 iron correct symbols 1 zinc 1 (c) can be bent (easily) or malleable or can be joined easily do not credit just can be joined not reactive or does not corrode or does not react (with hot water) accept does not rust 2 Page 25 of 34 (d) (i) chlorine do not credit chloride 1 (ii) one / 1 same number / amount 1 [10] M12. (a) A – electron 1 B – nucleus 1 C – proton 1 D – neutron 1 (b) Group 1 / alkali metals 1 has one electron in outer shell accept 3 protons / 3 electrons / atomic number 3 therefore lithium (so Group 1 / alkali metals) 1 (c) lithium accept Li 1 [7] M13. (a) 9 protons /Proton Number 9 mass / atomic number is neutral 1 10 neutrons 1 electron arrangement 2,7 / 9 electrons incorrect configurations neutral if no points scored, allow 1 mark for nucleus surrounded by electrons or nucleus contains neutrons and protons 1 Page 26 of 34 (b) Mark is for 2,8,2 arrangements. accept electrons anywhere in correct orbit 1 [4] M14. (a) less dense than air no marks if four or five box 1 very unreactive maximum 1 mark if three boxes ticked 1 (b) CO2 1 HCl 1 NH3 do not penalise upper / lower case or superscript 1 [5] M15. B carbon monoxide 1 CO accept carbon oxide do not credit carbon dioxide do not credit if any superscripts or subscripts used but accept C1O1, accept OC do not credit if obviously lower case 1 Page 27 of 34 C water 1 H2O accept hydrogen oxide do not accept hydrogen hydroxide do not credit if obviously lower case or if 2 not subscript do not accept HOH accept OH2 1 D ammonia 1 NH3 do not accept ammonium do not credit if obviously lower case, or if 3 not subscript accept nitrogen hydride or hydrogen nitride do not accept hydrogen nitrate or nitrite allow H3N 1 [6] M16. (a) light / caloric (heat) for 1 mark 1 (b) (i) lime (calcium oxide) / magnesia (magnesium oxide) barytes (barium sulphate) /argilla (aluminium oxide) / silex(silicon dioxide) for 1 mark 1 (ii) Lavoisier / he could not break it down into anything simpler by chemical methods of the time for 1 mark 1 [3] M17. electron nucleus neutron each for 1 mark [3] Page 28 of 34 ## (a) potassium, hydrogen, carbon, oxygen, chlorine or iodine 3 correct gains 1 mark 4 correct gains 2 marks all correct gains 3 marks (deduct 1 mark for each incorrect answer) 3 (b) potassium (K) for 1 mark 1 [4] ## (a) elements: aluminium, copper, compounds: pure water, sodium chloride, mixture: beer, milk 2/3 correct gains 1 mark 4/5 correct gains 2 marks all correct gains 3 marks 3 (b) metals: can be hammered into shape, good conductor of electricity, shiny non metals: brittle, dull, poor conductors of electricity 2/3 correct gains 1 mark 4/5 correct gains 2 marks all correct gains 3 marks 3 [6] M20. (a) lead oxide + carbon = lead + carbon dioxide (A symbol equation was accepted if correct) 1 (b) oxygen removed (or addition of electrons) 1 [2] M21. (a) a substance which contains one type of atom or a substance that cannot be broken down into anything simpler for 1 mark 1 Page 29 of 34 (b) more than one element/more than one type of atom combined/join together/bonded for 1 mark each 2 [3] M22. (a) (i) proton 1 (ii) neutron 1 (iii) nucleus 1 (b) there are shells or energy levels or orbitals do not accept ring 1 the maximum number of electrons found in the first shell or energy level is 2 accept first shell is full with 2 electrons 1 [5] M23. (a) (i) B 1 (ii) D 1 (b) A and B – only one type of atom 1 C and D – more than one type of atom accept element for atom ignore the word ‘mixture’ 1 (chemically) bonded accept (chemically) joined or similar idea of joined 1 [5] M24. (i) two circles together and shaded i.e. one molecule 1 Page 30 of 34 (ii) made up of one type of atom accept made up of atoms which contain the same number of protons accept a substance that cannot be split up into simpler substances by chemical means do not accept they are in the Periodic Table 1 (iii) no marks can be awarded if there is any reference to mixture or mix made up of two or more types of atoms accept made up of two or more elements 1 (chemically) bonded accept joined or combined for bonded do not accept fused 1 [4] M25. (a) fractional distillation 1 boiling point or use 1 (b) (i) mixture: compounds or elements or substances together but not chemically combined ignore references to separation 1 compound: (different) elements or different atoms together and chemically combined ignore references to separation 1 (ii) element: contains only one type of atom accept made of atoms which contain the same number of protons 1 compound: contains different types of atom chemically combined ‘chemically combined’ not needed here if already stated in (b)(i) 1 [6] M26. (a) (i) all correct two marks one or two correct one mark electron proton neutron 2 Page 31 of 34 (ii) (argon has) a full outer shell (of electrons) accept energy level for shell accept does not lose or gain electrons do not accept does not form bonds or react or is a noble or inert gas 1 (b) oxygen would react (with metal) accept oxygen is reactive do not accept metal would react (neutral) 1 metal would burn accept metal would be ‘destroyed’ or metal oxide formed or metal is oxidised do not accept it would explode or would not last long accept filament for metal 1 [5] M27. (i) nucleus 1 (ii) they both have seven electrons in the outer shell accept they both have the same number of electrons in the outer shell both need one electron to make full outer shell 1 [2] M28. (a) nucleus 1 electron 1 (b) correct number of electrons (12) accept dots and circles 1 2.8.2 1 [4] Page 32 of 34 M29. (a) react with oxygen / oxidise / burn in oxygen / burning / combustion or tungsten to tungsten oxide or makes an oxide key idea is oxidation ignore breaking ignore fire / flames / exothermic ignore react with air 1 (b) it is (very) unreactive / not reactive / inert / does not react with tungsten or it is a noble gas or it is in group 0 or 8 or 18 do not accept unreactive / inert metal or argon is not very reactive 1 full outer shell (of electrons) / 8 electrons in outer shell 1 does not need to gain / lose / swap / transfer / share electrons or does not need to form bonds does not bond ionically / covalently 1 [4] M30. (a) react with oxygen / oxidise / burn in oxygen / burning / combustion or tungsten to tungsten oxide or makes an oxide key idea is oxidation ignore breaking ignore fire / flames / exothermic ignore react with air 1 (b) it is (very) unreactive / not reactive / inert / does not react with tungsten or it is a noble gas or it is in group 0 or 8 or 18 do not accept unreactive / inert metal or argon is not very reactive 1 full outer shell (of electrons) / 8 electrons in outer shell 1 does not need to gain / lose / swap / transfer / share electrons or does not need to form bonds does not bond ionically / covalently 1 [4] Page 33 of 34 Page 34 of 34