The Silicon Environment in Silica Polymorphs, Aluminosilicate

The Silicon Environment in Silica Polymorphs,
Aluminosilicate Crystals and Melts: An In Situ High
Temperature XAS Study
L. Cormier
1
, D. R. Neuville
2
, J. Roux
2
, D. de Ligny
3
A.-M. Flank
5
, P. Lagarde
5
, G. S. Henderson
4
,
1
IMPMC, Université Pierre et Marie Curie, Université Denis Diderot, CNRS UMR 7590, 140 rue de Lourmel,
75015 Paris (France)
2
Physique des Minéraux et des Magmas, CNRS, IPGP, 4 place Jussieu, 75005 Paris (France)
3
LPCML, UCBL, 12 rue Ampère, 69622 Villeurbanne,( France)
4
Department of Geology, University of Toronto, 22 Russell Street, Toronto, M5S 3B1 Ontario (Canada)
5
Synchrotron SOLEIL, L'Orme des Merisiers, BP48, 91192 Gif s/Yvette cedex (France)
Abstract.
High temperature X-ray absorption spectroscopy at the Si K -edge has been used to obtain in situ information on SiO
2
phase transitions upon heating. Important modifications are observed for the XANES spectra of the high temperature polymorphs, in relation to disordering of the SiO
4
tetrahedra beyond the short-range correlations. This paper also presents the XANES spectra of anorthite (CaAl
2
Si
2
O
8
) from room temperature up to the melt (1900 K). This study shows the possibilities for determining the Si environment in crystals and glasses up to the liquid state using in situ
XANES measurements.
Keywords: Structure, phase transitions, X-ray absorption spectroscopy
PACS: 61.20.-p - 61.10.Ht - 64.60.-i
INTRODUCTION
Silica, SiO
2
, exists in many different phases at ambient pressure, with phase transitions occurring upon heating:
α
-quartz to
β
-quartz (847 K) to HPtridymite (1140 K) to
β
-cristobalite (1743 K) prior to melting at 2000 K. All these structures are networks of corner-sharing SiO
4
tetrahedra but the structure and nature of the disorder in the high-temperature forms is still controversial. Neutron diffraction data have shown structural similarities for these silica polymorphs that can be interpreted in terms of the extent to which the structures allow the silica tetrahedra to rotate and move [1]. Si K -edge XANES spectra are sensitive to both the local and medium range ordering, and could add constraints on the transitions which can be thermal (dynamic) or static for these three-dimensional structures. Furthermore, comparison of these silica polymorphs with amorphous silica is of interest to understand the local orientational disorder frozen in glasses (SiO
4
rotation, intertetrahedral angles).
Recently, contactless levitation furnaces with laser heating have been developed on synchrotrons [2] but these systems are best suited for high temperature heating above 1700 K. The heating device used for the present X-ray absorption measurements is a homemade setup consisting of a Pt-Ir10% wire, allowing access to temperatures from 300 K up to more than
2000 K. Measurements were carried out on the LUCIA beamline installed on the Swiss Light Source [3].
In situ XANES spectra at the Si
EXPERIMENTS
K -edge were acquired for the different SiO
2
polymorphs and crystalline anorthite (CaAl
2
Si
2
O
8
). Important region which is characteristic of medium range organization [4]. These results open new horizons for obtaining in situ information at high temperature over a wide range of temperatures.
XAS measurements were made using the LUCIA beam-line at SLS (Switzerland). This line is dedicated to micro X-ray absorption spectroscopy in the 0.8-
8 keV energy range. We have used a monochromator with KTP crystals for experiments at the Si diode detector protected by a beryllium cap.
In Situ Si Environment in Silica
Polymorphs
K
The home-made micro furnace (Pt-Ir10% or Ir heater) is described in more detail elsewhere [5] and was recently used to obtain XAS spectra of transition elements [6]. It was adapted to fit within the vacuum chamber. The temperature was measured using an optical pyrometer which analyzes a small surface of about 0.2 mm
2
. The beam size was set to 20*20 μ m.
The small size of the sample (800
μ m in diameter) reduces temperature gradients. The XAS spectra were recorded in the fluorescence mode using a silicon drift
RESULTS AND DISCUSSIONS
edge.
In SiO
2
polymorphs, Si in tetrahedral sites exhibits various degrees of medium range order, depending on the connection between the SiO
4
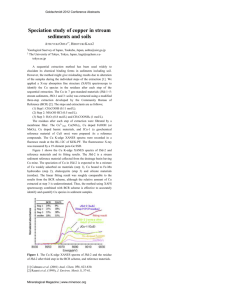
tetrahedra. The ability of Si K -edge XANES spectra to trace changes in medium range order is shown in Figure 1 by comparing spectra obtained at different temperatures.
The Si XANES spectra vary significantly with temperature, following the sequential transitions of the
SiO
2
polymorphs.
4
1873 K (
β
-cristobalite T range)
3.5
3 1373 K (tridymite T range)
2.5
2
1.5
873 K (
β
-quartz)
773 K ( α -quartz)
300 K (
α
-quartz)
1
0.5
A
B
C
D E
0
1840 1860 1880 1900
E (eV)
1920 1940 1960
FIGURE 1.
Si K -edge XAS spectra of SiO
2
as a function of temperature.
At room temperature, the spectrum for α -quartz is in agreement with previously reported data [7]. It can be described by a strong asymmetric absorption band at 1852 eV (peak A), which is related to tetrahedral coordination. At higher energies, three resonances (B,
C, ad D) are observed between 1850-1860 eV and a broad feature (E) exists at 1870 eV. Multiple scattering calculations have revealed that the structures B, C and
D are intimately related to medium range organization while structure A and E appear in a 5-atom cluster and are thus associated with the tetrahedral Si site [4,7].
As the temperature increases, SiO
2
undergoes phase transitions that induce modifications of the
XANES spectra. The
α
->
β
quartz transition exhibits only small variations in the relative intensity of the features in the 1850-1860 eV region. This displacive phase transition has been recently associated with the excitation of many low-frequency vibration modes which create considerable orientational disorder of the
SiO
4
tetrahedra [8].
At 1373 K, SiO
2
is in the temperature range of tridymite. The peaks B, C and D merge in a unique feature and the peak E shifts to lower energy by
1862 eV become broader in the temperature range of
β
-cristobalite at 1873 K. The white line (peak A) decreases in intensity, especially in β -cristobalite.
Associated with this decrease, is the appearance of a new peak at low energy. However, it is not clear if this peak is a real feature or originates from self absorption effects (these seem to be amplified within the wire due to the difficulty in orientation between the incident beam, the sample in the hole of the wire and the detector).
These high temperature SiO
2
phases are characterized by the tilting of the SiO
4
tetrahedra away from their ideal orientations, and the generation of rotational modes without significant distortion of the tetrahedra [9]. The disordering in the positions of the
SiO
4
tetrahedra is clearly responsible for the loss of the well defined peaks B, C and D. The XANES spectra of amorphous SiO
2
is qualitatively similar to β cristobalite, though the feature at 1852 eV is less pronounced [7]. This is consistent with the close similarity of the neutron total correlation functions observed between silica glass and tridymite and β cristobalite [1]. It was concluded that regions of size
~7.5 Å have similar structural arrangements, though they are too small to be microcrystals. This length scale agrees with the size of the clusters used in MS calculations to describe the 1850-1860 eV region in Si
K-edge spectra [4,7].
Si Environment in Anorthite Melt
The Si K -edge of anorthite (CaAl
2
Si
2
O
8
) was studied in the crystalline and molten state at 1900 K and the spectra are reported in Figure 2. In this composition, Si is localized in four-fold coordinated sites in a fully polymerized network, similar to SiO
2
, but connected with both SiO
4
and AlO
4
tetrahedra
[10]. Ca is localized near the (AlO
4
)
-
tetrahedra to
ensure charge compensation, in distorted sites with about 6-7 oxygen neighbors [10].
Apart from the intensity of the white line, the room temperature spectrum exhibits similarities to that of
β
cristobalite with two features at ~1852 (peak b ) and
1862 (peak c ) eV. Peak b is related to medium range organization and is less well defined than in SiO
2
quartz due to the presence of two types of second nearest neighbors (Si or Al in tetrahedral position). As the temperature increases, peak a broadens and peak b disappears. These changes could be due to modifications in the Si-O-Al angles [11]. We also note a shift to lower energy of ~2 eV in the position of the absorption edge during the melting of the samples, giving a direct measurement of the modification of the electronic structure. Such a shift has been previously observed in the melting of Y
2
O
3
at the Y K -edge and was interpreted as a decrease in the band gap with increasing temperature [2].
2.8
2.4
2
1.6
1.2
0.8
0.4
0
1900K
1830K
1000K
300K
1840 a b c
CONCLUSIONS
CaAl
2
Si
2
O
8
T l
=1826K
1860
E (eV)
1880 1900
FIGURE 2.
Si K -edge XAS spectra of anorthite
(CaAl
2
Si
2
O
8
) crystal and melt.
We have shown that, using the micro beam available at the LUCIA beamline, we can obtain Si K edge XANES spectra up to the liquid state, using a home-made Pt-heating device applicable to a very wide range of temperatures. The examples presented here show the potential for in-situ studies of phase transitions and for obtaining new insights into the structural reorganization on going from the crystalline to the liquid states.
ACKNOWLEDGMENT
This work was performed at the Swiss Light
Source, Paul Scherrer Institut, Villigen, Switzerland.
We are grateful to the machine and beamline groups whose outstanding efforts have made these experiments possible.
REFERENCES
1. D. A. Keen and M. T. Dove, Mineral. Mag.
64 , 447-457
(2000).
2. C. Landron, L. Hennet, L., T. E. Jenkins, G. N. Greaves,
J.-P. Coutures and A. K. Soper, Phys. Rev. Lett. 86 ,
4839-4842 (2001).
3. A.-M. Flank, G. Cauchon, P. Lagarde, S. Bac, M.
Janousch, R. Wetter, J.-M. Dubuisson, M. Idir, F.
Langlois, T. Moreno and D. Vantelon, Nucl. Instrum.
Meth. B 246 , 269-274 (2006).
4. D. Cabaret, M. Le Grand, A. Ramos, A.-M. Flank, S.
Rossano, L. Galoisy, G. Calas and D. Ghaleb, J. Non-
Cryst. Solids 289 , 1-18 (2001).
5. B. O. Mysen and J. D. Frantz, Chem. Geol.
96 , 321-332
(1992); P. Richet, P. Gillet, A. Pierre, M. A. Bouhifd, I.
Daniel and G. Fiquet, J. Appl. Phys.
74 , 5451-5456
(1193).
6. V. Magnien, D. R. Neuville, L. Cormier, B. O. Mysen,
V. Briois, S. Belin, O. Pinet and P. Richet, Chem. Geol.
213 , 253-263 (2004); V. Magnien, D. R. Neuville, L.
Cormier, J. Roux, J-L. Hazemann, O. Pinet and P.
Richet, J. Nucl. Mater.
352 , 190-195 (2006).
7. V. Briois, P. Sainctavit, A.-M. Flank, Jpn. J. Appl. Phys.
32 , 52-54 (1993); J. Z. Wu, F. Jollet and F. Seifert, J.
Phys.: Condens. Matter 10 , 8083-8092 (1998).
8. M. G. Tucker, M. T. Dove and D. A. Keen, J. Phys.:
Condens. Matter 12 , L723-L730 (2000).
9. M. G. Tucker, M. P. Squires, M. T. Dove and D. A.
Keen, J. Phys.: Condens. Matter 13 , 403-423 (2001).
10. L. Cormier, D. Ghaleb, D. R. Neuville, J-M. Delaye and
G. Calas, J. Non-Cryst. Solids 332 , 255-270 (2003); D.
R. Neuville, L. Cormier and D. Massiot, Geochim.
Cosmochim. Acta 68 , 5071-5079 (2004).
11. Z. Wu, C. Romano, A. Marcelli, A. Mottana, G. Cibin,
G. Della Ventura and G. Giuli, Phys. Rev. B 60 , 9216-
9219 (1999).