Coordination Compounds
advertisement

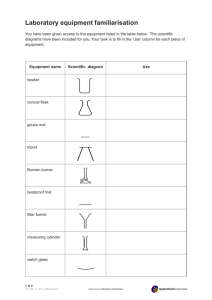
Coordination Compounds Introduction The transition metals form a large number of substances which consist of a transition metal atom or ion covalently bonded to several anions or neutral molecules. These substances are called coordination compounds, or complex ions. The attaching anions or neutral molecules are called ligands. The chemistry of the coordination compounds is rich because of the variety of geometries, colors, reactivities, and biological significance they display. The tetramminecopper(II) ion The nitrogen in ammonia covalently bonds to the copper using its unbonded pair of electrons. Four ammonia molecules arrange themselves about the copper ion in a square planar geometry. The copper has a 2+ charge in this substance. The copper complex will be crystallized out of solution as the sulfate salt. There is a set of nomenclature rules worked out for these compounds that leads to names which are often impressive. The two compounds in today’s lab are good examples of the naming rules. experiment Supplies From your drawer • 2 large test tubes • test tube rack • 1-250 ml beaker • 1-150 ml beaker for waste • 1 glass 50 ml beaker • 1 plastic 50 ml beaker • 1 watch glass • 1 stirring rod • 1 rubber policeman • 1 funnel From the cart • 2 sample containers • 2 pieces of filter paper You will synthesize two of these compounds today. One of them is a type of coordination compound called a chelate. In a chelate, the ligand has more than one point of attachment to the metal atom or ion. The ligand is called polydentate (“many toothed”). Three oxalate ions attach to an iron ion to give: 3- O O OC O CO O Fe C O CO O C O C O O Part 1. Potassium tris(oxalato)ferrate(III). The reaction is FeCl3 + 3 K2C2O4 → K3[Fe(C2O4)3] + 3 KCl All of the substances are in aqueous solution. You will allow the green complex iron salt to precipitate by forming a mixed solvent. In this case, the mixed solvent is created by adding ethanol to a water solution of the iron complex. When the complex ion salt precipitates, it carries three molecules of water with it, so that the solid crystals have the formula: K3[Fe(C2O4)3]·3H2O. The tris(oxalato)ferrate(III) ion The oxalate ion, C2O42-, has two points of attachment to the iron ion, so it is bidentate. Three oxalate ions arrange themselves about the iron ion in an octahedral geometry. Note that the iron ion must have a 3+ charge in this substance in order to account for the 3- charge on the ion. The iron chelate will be crystallized out of solution as the potassium salt. The other coordination compound you will prepare has a unidentate ligand, ammonia, attached to a copper ion in four positions: 1 While you are making the iron compound, you will also prepare a solution of copper sulfate that will be used in Part 2 of the experiment. 15 minutes, add an inch of ice to a 250 ml beaker and set the 50 ml beaker on top of the ice. Rub the stirrer across the inside bottom of the beaker a few times. This can seed the solution with nanometer sized chunks of glass that often initiate crystal growth. The crystals should grow for at least 30 minutes. Set up a hot water bath as shown: While you are waiting, go on to Part 2 of the experiment. Then decant the liquid from the crystals into the waste beaker. Add 2 ml of ethanol to rinse the crystals. Swirl, and decant the liquid from the rinse into the waste beaker. Use the rubber policeman to transfer the crystals onto a piece of filter paper setting on a double folded piece of paper towel. Spread the crystals out and allow to dry. Weigh the crystals on a piece of weighing paper. Put them in the container provided and keep them. Label the container with the formula and weight of the substance, as well as your name and the date. Use ink on white tape for the label. Show the sample to the instructor when you turn in your lab. Discard liquid from the waste beaker into the container in the fume hood marked “Iron Waste”. 250 ml beaker The iron compound is light sensitive. Over time, if kept in the light, it will turn a rusty brown. Part 2.Tetramminecopper(II) sulfate a) Add water to the beaker to about the 150 ml mark. Heat the water in the beaker to boiling. Once it boils, reduce the flame to low heat so that the water simmers. The chemical reaction is CuSO4 + 4 NH3 → [Cu(NH3)4]SO4 + 4 H2O All of the substances are in water solution. You will allow the deep blue complex copper salt to precipitate by forming a mixed solvent. In this case, the mixed solvent is created by adding ethanol to a water solution of the copper complex. When the complex ion salt precipitates, it carries one molecule of water with it, so that the solid crystals have the formula: [Cu(NH3)4]SO4·H2O. For Part 2 of the experiment, weigh out 1.0 gram of CuSO4. 5H2O on a tared piece of paper. First fold the paper to put a crease in it, and then unfold it. The crease will help to pour the solid into a test tube. Add the solid to a test tube. Add 2.5 ml of deionized water to the test tube and place it in the hot water bath. Pour the blue solution prepared in Part 1 from the test tube into a plastic 50 ml beaker. While working in the fume hood, add concentrated NH3 solution, a milliliter or so at a time, stirring constantly. At first, a precipitate of Cu(OH)2 forms. As more NH3 is added, the initial precipitate dissolves and a clear, deep blue solution forms. The color is intense, which makes it difficult to see if all of the solid has gone into solution. Use the stirring rod to pull some of the liquid up the side of the beaker, and try to see if any specks of solid remain in the thin film of liquid that forms. If any solid remains, add more NH3. For Part 1, use the transfer pipets on the reagent bottles to add 1 ml of 1 M FeCl3 solution and 2 ml of a saturated K2C2O4 solution to the other test tube and place the test tube in the hot water bath. Leave it in the bath for 5 minutes. Stir the contents of the copper sulfate test tube for Part 2 until all of the solid is dissolved, then remove the test tube from the bath and set it in the test tube rack. Turn the bunsen burner off. Use the transfer pipet on the reagent bottle to add 1.5 ml of ethanol to the 50 ml glass beaker. Do not bring ethanol to your work station until the burner is off. Pour the hot, green contents of the test tube still in the hot water bath into the beaker and stir. Put the watch glass on the beaker and set the beaker out of the way. The watch glass goes on curved side down. Crystals should form while you work on Part 2. If no crystals appear within Add 2 ml of ethanol to the solution and stir well. The copper compound is much less soluble in the mixed water/ethanol solvent than it is in water. Fold a piece of filter paper and place it in the funnel as shown on the next page: 2 the instructor for help using a filtering aid. After this drains through, add 2 more ml of ethanol to the beaker, then pour it into the funnel. After the second addition drains through, pull the filter paper from the funnel, set it on a double thickness of paper towel, then pat it as dry as possible with paper towel. Finally, spread the solid out in a thin layer on a fresh double thickness of paper towel to dry. Use a spatula to chop the solid mass into a powder-like layer. Pour the liquid from the filtering into the waste container in the fume hood marked “Copper waste”. Fold paper in half, then fold again Pull 3 thicknesses over to make a cone Do #1 to 4 and 6 to 9 on the data table while you are waiting for the material to dry. When dry, weigh on a piece of weighing paper. Put the crystals in the container provided and keep them. Label the container with the formula and weight of the substance, as well as your name and the date. Show the vial to the instructor. Place the paper into the funnel, then moisten the paper with deionized water. Pour off excess water. Press the paper into the funnel to give a tight fit. The ammonia molecules are loosely attached to the copper, and over time, the color of the compound will fade. test tube Use a rubber policeman to transfer the solid/liquid mass to a filter paper in a funnel inserted in a test tube. After the liquid finishes running out, add 2 ml of ethanol to the beaker, and stir to mix any solid remaining in the beaker into the liquid, and then pour this onto the solid in the filter paper to rinse it. If the liquid is slow to drain out of the funnel, ask 1. 2. Volume of 1 M FeCl3 solution used DAT A, Parts 1 and 2 ml Moles of FeCl3 used mol 3. Moles of K3[Fe(C 2O4)3]·3H2O theoretically produced mol 4. Grams of K3[Fe(C 2O4)3]·3H2O theoretically possible g 5. Grams of K3[Fe(C 2O4)3]·3H2O actually produced g 6. Mass of CuSO4·5H2O used g 7. Moles of CuSO4·5H2O used mol 8. Moles of [Cu(NH3)4]SO4·H2O theoretically produced mol 9. Grams of [Cu(NH3)4]SO4·H2O theoretically possible g 10. Grams of [Cu(NH3)4]SO4·H2O actually produced g 3 Name_________________________________________ Grade___________ Date ___________ Questions 1. Describe the physical appearance of each complex substance produced. (Instructor’s OK after viewing samples in clearly labeled containers: Initials _________) 2. Percent yield is calculated by dividing the grams obtained by the grams possible, then multiplying by 100. Calculate the percent yield for each complex ion. Note that this is not a % difference calculation. Part 1: Part 2: 3. There are often side reactions taking place that reduce the % yield of a substance. How do you account for yields in excess of the theoretical yield? 4