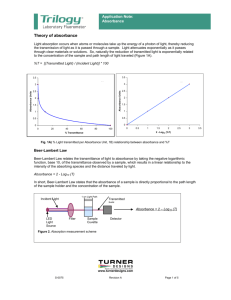
REPORT 5
advertisement
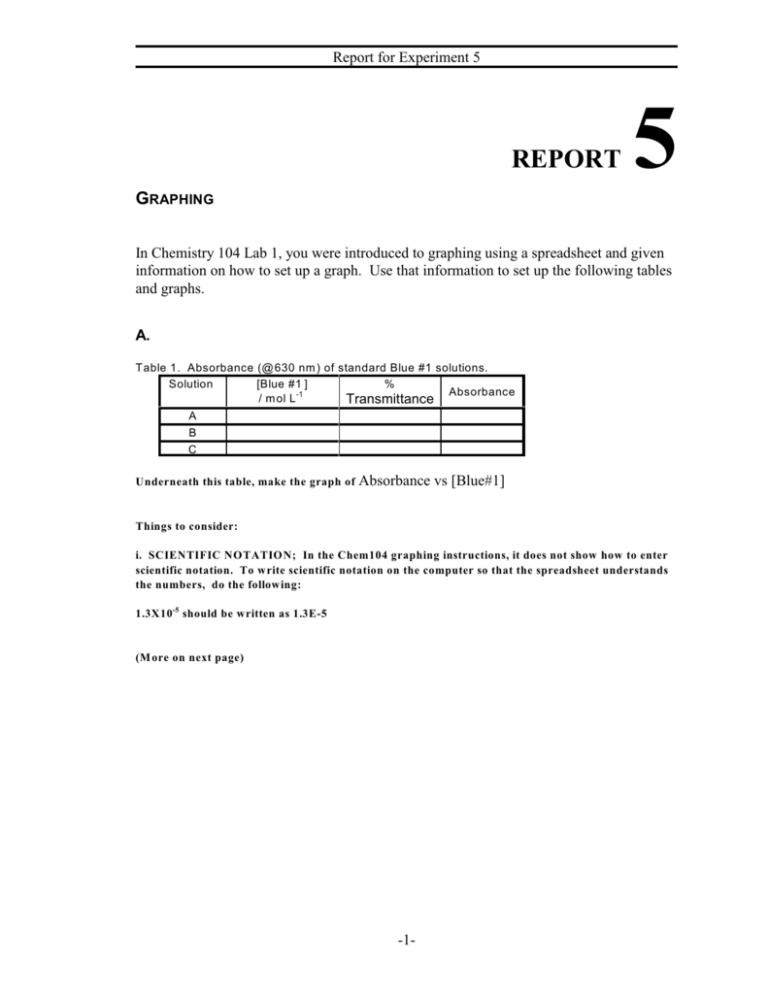
Report for Experiment 5 REPORT 5 GRAPHING In Chemistry 104 Lab 1, you were introduced to graphing using a spreadsheet and given information on how to set up a graph. Use that information to set up the following tables and graphs. A. Table 1. Absorbance (@630 nm ) of standard Blue #1 solutions. Solution [Blue #1 ] % Absorbance / m ol L -1 Transmittance A B C Underneath this table, make the graph of Absorbance vs [Blue#1] Things to consider: i. SCIENTIFIC NOTATION; In the Chem104 graphing instructions, it does not show how to enter scientific notation. To write scientific notation on the computer so that the spreadsheet understands the numbers, do the following: 1.3X10-5 should be written as 1.3E-5 (M ore on next page) -1- Report for Experiment 5 ii. EQUATIONS: In the Chemistry 104 graphing instructions, it shows how an equation can be set up. You may think it is easier to just calculate this yourself, but letting the computer do things for you can save you a lot of time. So we want you to learn this. The first equation you can try is to for % Transmittance to be converted to Absorbance. M athematically, we know In the above, we need to write this so that the computer understands the formula. Let’s try putting the formula in E6. Our % T values will be in cell D6. Remember that an “=” sign should be placed at the beginning so that the computer knows you are doing a formula. In E6: =-LOG(D6/100) You can now copy the formula from E6 and paste them in cells E7-E8. -2- Report for Experiment 5 W ith our name and date, our data and the first graph underneath, we have completed the first graph page. Remember that a descriptive title should include information like the wavelength and the material (FD&C Blue#1). The graph should be large enough to be easily read. (Second graph page instructions on next page) -3- Report for Experiment 5 Part B. In the second graph page we should set up a table like this for Solution B: Here I have already inserted a formula in D7 to calculate the Absorbance values from % T. To convert your absorbance values to concentration values, use your 1st graph (Absorbance vs Concentration from Part A) This graph from Part A will give you an equation in the form of y=mx+b Let’s get the computer to calculate the concentration (Cell E7) from your absorbance value (cell D7). Say from your working graph (Absorbance vs Concentration), you had an equation y=137000x+0.0062 Since y values are the absorbance values, we need to solve for the x values (concentration). Therefore y=mx+b y-b=mx (y-b)/m=x y=137000x + 0.0062 y - 0.0062 = 137000x (y - 0.0062) / 137000 = x So we need to solve for x with the formula “(y-b)/m”. To write this formula in E7, we would write =(D7-0.0062)/137000 remember the “=” sign This would calculate the concentration in Cell E7. This formula can then be copied and pasted below. To get the natural log (LN) of the concentration in cell E7, we would write the formula in F7. =LN(E7) Try to think of a formula for cell G7. -4- Report for Experiment 5 Now make up the 3 graphs for the zero, 1st , and 2nd order kinetics. In other words, make a graph of “Concentration vs time”, “LN Concentration vs time”, and “1/Concentration vs time”. You should be able to fit the data and the 3 graphs on 2 pages. For these graphs, have the computer graph a best fit straight trend line (do not choose a curved trendline). You can save this file. In the last part, we need to make up a graph page for Solution C. This should not be hard. Take your graph page from Solution “B”, and now save it with a different file name. If you enter your data from Solution C, over the old data from Solution B - the computer will automatically compute your data and fix your graphs accordingly. (This only works if you actually used formulas when making up your previous graph.) If you just change your titles so it is clear that this is data from Solution C, you will have the table and graphs finished quickly. -5-