SLMP - FMC Lithium
advertisement

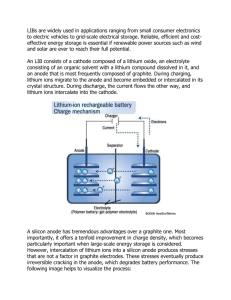
Stabilized Lithium Metal Powder (SLMP™) – Material and Application Technologies for High Energy Li-ion Batteries Marina Yakovleva, K.B. Fitch, Yangxing Li and Yuan Gao www.fmclithium.com Contact info: marina.yakovleva@fmc.com 26th International Battery Seminar & Exhibit, March 16th-19th, 2009 Market Needs • New electrode materials are required to increase energy density of Li-ion batteries • Li-ion technology has expanded into large format batteries • Automotive use demands lower cost and improved safety – Need more choices for active and inactive materials! – Need to break the current limitation that all lithium has to come from the cathode of the Li-ion cell. 2 26th IBS&E, March 16-19th, 2009 Lectro® Max 100 Series, Stabilized Lithium Metal Powder • Normal lithium powder – Can only be handled in an argon filled glove box – Not commercially available as powder • Stabilized Lithium Metal Powder (SLMP™) SEM Image of SLMP – Safe to handle in a dry room – Can be transported by air or sea – Metallic Li content is at least 98% • FMC has been producing lithium powder for its own use at the level of thousands of tons/year for over 30 years Optical Microscope Image of SLMP sprayed on the electrode 3 26th IBS&E, March 16-19th, 2009 It is a Li-ion System • The anode host material is lithiated with SLMP to produce a true Li-ion anode when electrolyte is added at cell activation. • SLMP provides an independent source of Lithium and it combines the benefits of the two past systems, which enables many possibilities for energy and performance enhancements – Li metal cell : non-lithium providing cathode – Li-ion cell: host anode material that intercalates lithium There is NO metallic Li left after cell is activated 4 26th IBS&E, March 16-19th, 2009 Benefits of SLMPTM Opening up choices for active materials – Anode choice no longer limited to graphite. • Allows the use of new materials with both large reversible and irreversible capacities, such as Si composites and Sn intermetallics. – Cathode choice no longer limited to lithium providing materials. • Much wider selections of non lithium providing materials offering more possibilities: more overcharge tolerant, lower cost, and larger capacities. – Use of SLMP™ in battery material synthesis • Si and Sn Composite anode materials Bottom line: increase in energy density, improvements in safety and calendar life, cost reductions Using Lithium from SLMP will cost less than using Lithium from LiCoO2 cathode and you can pocket other advantages too 5 26th IBS&E, March 16-19th, 2009 A Cathode Example, MnO2 A Li-ion cell: EMD/Graphite+SLMP ™ Specific Capacity, (discharge mAh/g) 300 200 100 Constant Current 0.1mA, Charge 4.3V, Disharge 1.5V 0 0 5 10 15 20 Cycle Number 6 26th IBS&E, March 16-19th, 2009 25 30 THE STATE UNIVERSITY OF NEW JERSEY RUTGERS A Cathode Example, BiF3 A Li-ion cell: BiF3 Nanocomposite/Graphite+SLMP ™ 4 BiF 3.5 3 Cell Voltage 3 2.5 2 "Li 1.5 BiF " 3 vs.Li-1st dis vs.Li2nd dis vs.Li 3rd dis vs.LiMCMB-1st dis vs.LiMCMB 2nd dis vs.LiMCMB 3rd dis BiF Nanocomposite 3 LiPF EC:DMC 6 1 2.45 o 24 C 15 mA/g 0.5 0 2 4 6 8 10 12 Discharge Time (h) (Data courtesy of Rutgers University) 7 26th IBS&E, March 16-19th, 2009 14 16 An Anode Example First Cycle Efficiency Improvement in Commercial SiO Using SLMP 2.5 2.5 Voltage (V) Irreversible Capacity ~487 mAh/g 1.5 1.0 Voltage (V) 2.0 2.0 1.5 1.0 0.5 0.5 0.0 0.0 0 200 400 600 800 1000 1200 1400 1600 Irreversible Capacity ~155 mAh/g 0 200 600 800 1000 1200 1400 1600 Capacity (mAh/g) Capacity (mAh/g) Baseline Half cell: silicon composite material from Shin Etsu Corporation ™ (85%), PI binder (15%), GBL as a solvent 400 SLMP-treated silicon composite material from Shin Etsu Corporation (85%), PI binder (15%), GBL as a solvent Test Conditions: constant current charge/discharge, 0.0V to 1.5V 8 26th IBS&E, March 16-19th, 2009 Calendar Life Improvements Cycling Performance of the 5Ah cells (a) baseline cell (red line), (b) electrode treated with SLMP (blue line) with the loading of 1.2% of the MCMB weight, C/2 rate, 3-4.2V range. Important for long calendar life: SLMP serves as a “getter” of moisture and acidic species (Data courtesy of SKC PowerTech Corporation) 9 26th IBS&E, March 16-19th, 2009 SLMP™ Introduction into the Cell Two general methods to apply SLMP™ • Surface application – Coat an SLMP™ suspension on the surface of pre-fabricated anode sheet – no need to change the existing anode fabrication process • Slurry application – Include SLMP™ in the slurry mix when the anode sheet is being cast – no additional step but the slurry solvent needs to be compatible with lithium. 10 26th IBS&E, March 16-19th, 2009 CLEAR Center for Lithium Energy Advanced Research • Equipped for demonstration of safe handling of the SLMP Technology • Equipped for demonstrations of multiple SLMP Application Methods using customer’s electrodes • Equipped for making laminated full lithium-ion cells 11 26th IBS&E, March 16-19th, 2009 Surface Application Multiple methodologies have been evaluated: • • • • • • A B Spraying Dip Coating Doctor blade Coating Dry/Wet Sieving Printing painting Keeping SLMP uniformly distributed in the suspension is the key A: SLMP in non-polar solvent without additives, fast separation B: Additives keep SLMP suspended for extended time periods 12 26th IBS&E, March 16-19th, 2009 Spray Application Study-Demonstration (Anode) 13 26th IBS&E, March 16-19th, 2009 An Anode Example A Li-ion cell: LiCoO2/Graphite+SLMP ™ 4.5 4.0 Voltage (V) 3.5 3.0 Baseline First Cycle Discharge Graphite/LiCoO2 Cell 2.5 First Cycle Discharge SLMP+Graphite/LiCoO2 Cell 2.0 Improvement 1.5 1.0 0.0% 20.0% 40.0% 60.0% 80.0% 100.0% Capacity 14 First Cycle Efficiency Improvement in LiCoO2/Graphite System Using SLMP (Data courtesy of MaxPower Corporation) 26th IBS&E, March 16-19th, 2009 Spray Application Study-Demonstration (Separator) 15 26th IBS&E, March 16-19th, 2009 Optical Microscope Images of the Separator Film SLMP-treated Electrode SLMP-treated and pressed Electrode Open Circuit Voltage (V vs. Li) Carl Zeiss Axio Imager D1 digital imaging microscope, 100x magnification. Open circuit voltage for half-cells Li/graphite/glass paper/Celgard 3501 separator/graphite: a) Baseline cell (Vo=3.2V) b) SLMP-treated separator (Vo=0.14V) 3.0 2.0 Li/MCMB Cell 1.0 Baseline cell SLMP-treated separator 0.0 0 20 40 60 80 Time (hour) 16 26th IBS&E, March 16-19th, 2009 Industrially Scalable Process • Set-up designed for the Dcell production with SLMPTM Technology incorporated • under US Army contract W15P7T-06-C-P242. Unwind/Rewind Speed Control Large Area for spray application Exhaust Chamber fitted to rewind portion of machine B. Meyer and F. Cassel, M. Yakovleva and Y. Gao, and G. Au , 43rd Power Sources Conference, Philadelphia, Pennsylvania, July 7 – 10, 2008 17 26th IBS&E, March 16-19th, 2009 SLMPTM Surface Application (spray method) As sprayed After pressing 26th IBS&E, March 16-19th, 2009 18 SLMPTM Surface Application (spraying method) Prior to electrolyte addition Right after electrolyte addition, time=0 time=2 min 19 26th IBS&E, March 16-19th, 2009 Time =30 min SLMP Diffusion into Carbonaceous Anode 26th IBS&E, March 16-19th, 2009 20 Thin Li-foil Diffusion into C-Anode Rolled Li thin foil (<30 micron): macro view Thin Li foil as applied onto the surface of prefabricated anode After 7 days of storage at 25oC After 9 days of storage at 25oC 21 26th IBS&E, March 16-19th, 2009 Thin Li-foil Diffusion into C-Anode Prior to electrolyte addition after electrolyte addition, time=1hr time=1 day 22 time=7 days 26th IBS&E, March 16-19th, 2009 New Capabilities: Full Pouch Cells 23 26th IBS&E, March 16-19th, 2009 First Cycle Loss • SLMP Effect on Loss • Effect of Processing – Efficiency – Apparent versus actual concentration 10 5 First Cycle Loss (%) – Performs as if intercalated from Li metal – Optimum concentration Baseline Surfactant 0 0 1 2 3 Surfactant and Carbon Linear (Baseline) -5 Linear (Surfactant) Linear (Surfactant and Carbon) -10 -15 Lithium Concentration (%) 24 4 26th IBS&E, March 16-19th, 2009 Summary • More material choices are needed to meet the increasing demands for more energy, lower cost and better safety for Li-ion batteries. • SLMP™ provides an independent source of lithium for Li-ion batteries, which opens up the choices of both anode and cathode materials to meet such demands. • SLMP™ can be introduced into the cell through industrially scalable methods. • Lithium from SLMP will be at a lower cost than Lithium from LiCoO2 cathode. • SLMP™ could be customized to meet specific customer requirements • Progress in SLMP™ Application Technologies demonstrated We are welcoming the Battery Community to visit CLEAR and apply SLMP onto your current and advanced electrode materials and get trained in safe Lithium handling 25 26th IBS&E, March 16-19th, 2009 SLMP Sampling 26 26th IBS&E, March 16-19th, 2009 Acknowledgement Co-workers: – Scott Petit, Prakash Palepu, Yoshimasa Nomiyama, George Sandor Rutgers, The State University of New Jersey, ESRG: – Glenn Amatucci, Irene Plitz, Adam Skrzypczak, Fadwa Badway MaxPower: – Frank Cassel, David Chua, Benjamin Meyer US Army CERDEC: – George Au SKC PowerTech - Dr. Zhiwei Zhang, Dr. Zhiqiang Xu 27 26th IBS&E, March 16-19th, 2009