(TLC) System for NatuRose™ Carotenoids
advertisement

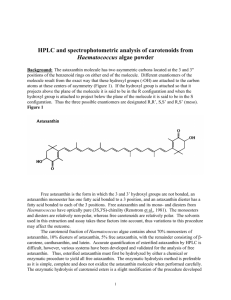
Thin-Layer Chromatography (TLC) System for NatuRose Carotenoids Background: The astaxanthin molecule has two asymmetric carbons located at the 3 and 3" positions of the benzenoid rings on either end of the molecule. Different enantiomers of the molecule result from the exact way that these hydroxyl groups (-OH) are attached to the carbon atoms at these centers of asymmetry (Figure 1). If the hydroxyl group is attached so that it projects above the plane of the molecule it is said to be in the R configuration and when the hydroxyl group is attached to project below the plane of the molecule it is said to be in the S configuration. Thus the three possible enantiomers are designated R,R’, S,S’ and R,S’ (meso). Figure 1 Free astaxanthin is the form in which the 3 and 3’ hydroxyl groups are not bonded, an astaxanthin monoester has one fatty acid bonded to a 3 position, and a astaxanthin diester has one fatty acid bonded to each of the 3 positions. Free astaxanthin and its monoand diesters from Haematococcus have optically pure (3S,3'S)-chirality (Renstrom et al., 1981). The mono-esters and di-esters are relatively non-polar, whereas free carotenoids are relatively polar. The solvents used in this extraction and assay takes these factors into account, thus variations to this procedure may effect the outcome. The carotenoid fraction of NatuRose Haematococcus algae meal contains about 70% monoesters of astaxanthin, 10% diesters of astaxanthin, 5% free astaxanthin, with the remainder consisting of β-carotene, canthaxanthin, and lutein. Quantitation of esterified astaxanthin by HPLC is difficult, however, various systems have been developed for the analysis of free astaxanthin. Esterified astaxanthin must first be hydrolyzed by either a chemical or enzymatic procedure to yield all free astaxanthin before proceeding with HPLC analysis. The enzymatic hydrolysis method is preferable as it is simple, complete and does not oxidize the astaxanthin molecule when performed carefully. NatuRose Technical Bulletin #015 describes the extraction, purification, hydrolysis and analysis of carotenoids by spectrophotometric and HPLC analysis. NatuRose Technical Bulletin #007 describes extraction and purification of carotenoids from finished feeds. Materials: Silica Gel Plates (Merck 60A pore size, 250 um thickness) Solvents: acetone and hexane TLC chamber Nitrogen manifold Glass beads (through 20 mesh) 50 ml (28.5 x 104mm) Nalgene Oakridge polypropylene copolymer centrifuge tubes (w/lids) Vortexer (Maxi Mix II, Fischer Scientific) Method: Note: All manipulations should be performed in low light and temperatures, as carotenoids are very sensitive to light, oxygen and heat. It is recommended that manipulations be performed in a darkened room while keeping the temperatures at 5 C if possible. Rf values will vary slightly, use standards to confirm the carotenoids. 1) Weigh approximately 10-15 mg of powder into a 50 ml centrifuge tube. 2) Add 5 grams of glass beads. 3) Add 2 ml of acetone to the centrifuge tube and vortex vigorously for 5 minutes. 4) Add an additional 5 mls of acetone, vortex briefly, and centrifuge tube for 5 minutes at 3000 rpm. 5) Pipette off the supernatant and transfer to a clean test tube. 6) Dry acetone extract down under nitrogen and re-suspend sample in 0.5 ml of petroleum ether or ethyl ether. Filtration is usually not necessary for TLC analysis. 7) Carefully spot the extract onto the TLC plate and allow to dry completely, do not overload silica gel. 8) Add a fresh mixture 25% acetone in hexane before each run (approximately 100 mls). Add the plate to the TLC chamber, and allow the TLC plate to develop for approx. 30 minutes. Remove from TLC chamber and quickly mark the solvent front and center of the carotenoid spots with a pencil. 9) Individual spots can be scraped off with a razor blade, minced in a test tube, and eluted with petroleum ether or ethyl ether for further analysis. Carotenoid β-carotene Echinenone Astaxanthin Diesters Astaxanthin Monoesters Canthaxanthin Astaxanthin Free Lutein Typical Rf value Rf=0.99 Rf=0.87 Rf=0.75 Rf=0.50 Rf=0.40 Rf=0.33 Rf=0.25 Grung M., F.M.L. D’Souza, M. Borowitzka, and S. Liaaen-Jensen. 1992. Algal carotenoids 51. Secondary carotenoids 2. Haematococcus pluvialis aplanospores as a source of (3S, 3'S)-astaxanthin esters. J. Appl. Phycol. 4: 165-171. Renstrom B. and S. Liaaen-Jensen. 1981. Fatty acid composition of some esterified carotenols. Comp. Biochem. Physiol. B., Comp. Biochem. 69: 625-627. NatuRose Technical Bulletin #003 Contact: Dr. R. Todd Lorenz Cyanotech Corporation Phone: 808-326-1353 FAX: 808-329-3597 Email: tlorenz@kona.net www.cyanotech.com Revision Date: November 24, 1998