Simplifying the Clinical Trial Supply Chain
Technology-enabled integrated Testing out new medicines in key markets worldwide and organizing consistent delivery,
tracking and management of trial-related supplies to multiple locations can become a
processes can provide greater
significant burden on resources. And as clinical trial supply chains become more complex,
visibility into each critical
the limitations of traditional management approaches are magnified.
supply chain stage. This helps
By utilizing a single point of contact for clinical trial supply logistics that combines intelligent
to achieve improved planning
processes with integrated technology enables you to identify opportunities, solve problems,
and supply monitoring, resulting and implement actions that optimize supply levels, reduce costs and enhance future trials.
in risk mitigation and reduced
Contact us today to find out more:
supply chain costs.
PAREXEL.com/simplify-chain or info@PAREXEL.com
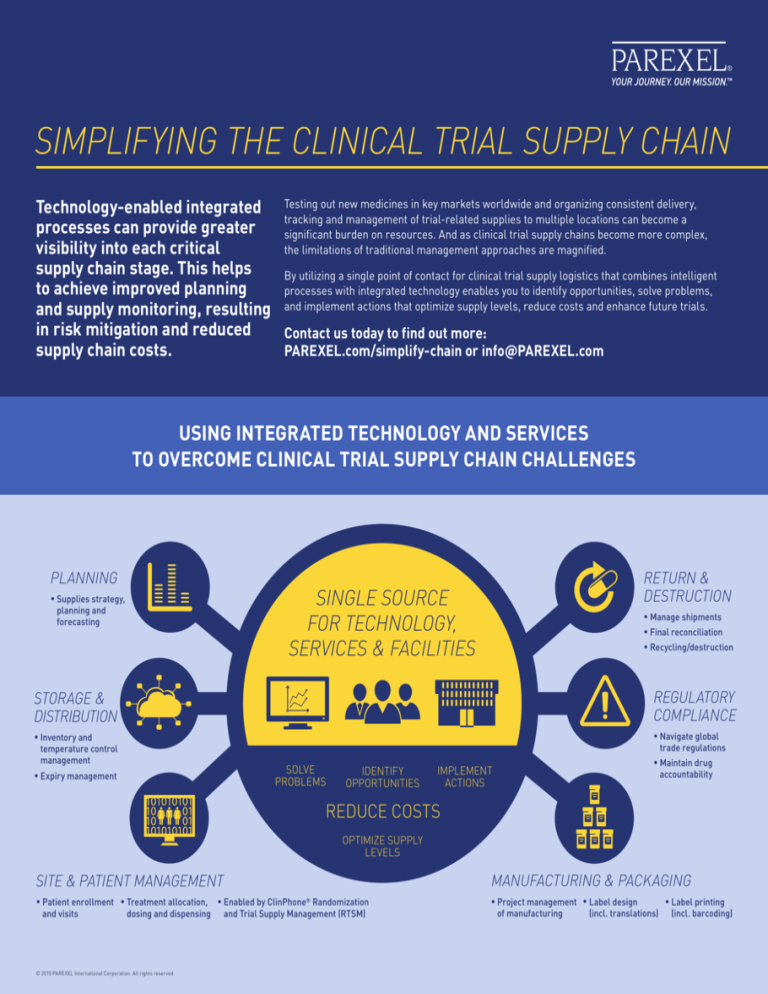
Using integrated technology and services
to overcome clinical trial supply chain challenges
Planning
•Supplies strategy,
planning and
forecasting
Return &
Destruction
SINGLE SOURCE
FOR TECHNOLOGY,
SERVICES & FACILITIES
•Manage shipments
•Final reconciliation
•Recycling/destruction
Storage &
Distribution
Regulatory
Compliance
•Inventory and temperature control
management
•Navigate global trade regulations
•Expiry management
SOLVE
PROBLEMS
IDENTIFY
OPPORTUNITIES
IMPLEMENT
ACTIONS
•Maintain drug
accountability
REDUCE COSTS
OPTIMIZE SUPPLY
LEVELS
Site & Patient Management
Manufacturing & Packaging
•Patient enrollment •Treatment allocation, •Enabled by ClinPhone® Randomization
dosing and dispensing
and visits
and Trial Supply Management (RTSM)
•Project management •Label design •Label printing of manufacturing
(incl. translations)
(incl. barcoding)
© 2015 PAREXEL International Corporation. All rights reserved.