Homework: Calculating with Chemical Equations
advertisement
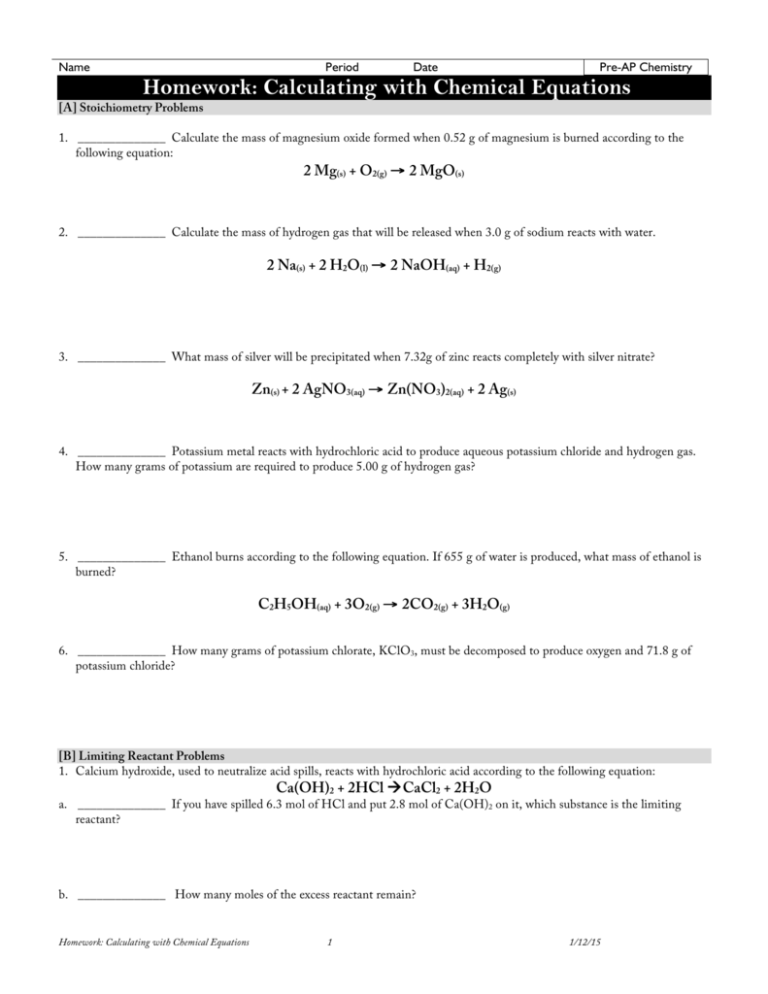
Name Period Date Pre-AP Chemistry Homework: Calculating with Chemical Equations [A] Stoichiometry Problems 1. ______________ Calculate the mass of magnesium oxide formed when 0.52 g of magnesium is burned according to the following equation: 2 Mg(s) + O2(g) → 2 MgO(s) 2. ______________ Calculate the mass of hydrogen gas that will be released when 3.0 g of sodium reacts with water. 2 Na(s) + 2 H2O(l) → 2 NaOH(aq) + H2(g) 3. ______________ What mass of silver will be precipitated when 7.32g of zinc reacts completely with silver nitrate? Zn(s) + 2 AgNO3(aq) → Zn(NO3)2(aq) + 2 Ag(s) 4. ______________ Potassium metal reacts with hydrochloric acid to produce aqueous potassium chloride and hydrogen gas. How many grams of potassium are required to produce 5.00 g of hydrogen gas? 5. ______________ Ethanol burns according to the following equation. If 655 g of water is produced, what mass of ethanol is burned? C2H5OH(aq) + 3O2(g) → 2CO2(g) + 3H2O(g) 6. ______________ How many grams of potassium chlorate, KClO3, must be decomposed to produce oxygen and 71.8 g of potassium chloride? [B] Limiting Reactant Problems 1. Calcium hydroxide, used to neutralize acid spills, reacts with hydrochloric acid according to the following equation: Ca(OH)2 + 2HCl àCaCl2 + 2H2O a. ______________ If you have spilled 6.3 mol of HCl and put 2.8 mol of Ca(OH)2 on it, which substance is the limiting reactant? b. ______________ How many moles of the excess reactant remain? Homework: Calculating with Chemical Equations 1 1/12/15 2. Aluminum oxidizes according to the following equation: 4Al + 3O2 à 2Al2O3 a. ______________ Powdered Al (0.048 mol) is placed into a container containing 0.030 mol O2. What is limiting reactant? b. ______________ How many moles of the excess reactant remain? [C] Percent Yield Problems 1. ______________ Dichlorine monoxide, Cl2O is sometimes used as a powerful chlorinating agent in research. It can be produced by passing chlorine gas over heated mercury (II) oxide according to the following equation. What is the percent yield, if the quantity of the reactants is sufficient to produce 0.86g of Cl2O but only 0.71 g is obtained? HgO + Cl2 àHgCl2 + Cl2O 2. In the commercial production of the element arsenic, arsenic(III) oxide is heated with carbon, which reduces the oxide to the metal according to the following equation: 2As2O3 + 3C à3CO2 + 4As a. ______________ If 8.87g of As2O3 is used in the reaction and 5.33 g of As is produced, what is the percent yield? b. ______________ If 67 g of carbon is used up in a different reaction and 425g of As is produced, calculate the percent yield of this reaction. 3. ______________ Huge quantities of sulfur dioxide are produced from zinc sulfide by means of the following reaction. If the typical yield is 86.78%, how much SO2 should be expected if 4897g of ZnS are used? 2ZnS(s) + 3O2(g) à2ZnO(s) + 2SO2(g) 4. ______________ A process by which zirconium metal can be produced from the mineral zirconium (IV) orthosilicate, ZrSiO4, starts by reacting it with chlorine gas to form zirconium (IV) chloride. What mass of ZrCl4 can be produced if 862g of ZrSiO4 and 950.g of Cl2 are available? (You must first determine limiting reactant). ZrSiO4 + 2Cl2 à ZrCl4 +SiO2 + O2 Homework: Calculating with Chemical Equations 2 1/12/15 Name Period Date Pre-AP Chemistry Homework: Calculating with Chemical Equations [A] Stoichiometry Problems 1. ______________ Calculate the mass of magnesium oxide formed when 0.52 g of magnesium is burned according to the following equation: 2 Mg(s) + O2(g) → 2 MgO(s) 0.52g Mg × 1mole Mg = 0.0214 mole Mg 24.31g × 2 MgO =0.0214 mole MgO × 40.31 g = 0.8626 = 0.86 grams of MgO 1moleMgO 2 Mg 2. ______________ Calculate the mass of hydrogen gas that will be released when 3.0 g of sodium reacts with water. 2 Na(s) + 2 H2O(l) → 2 NaOH(aq) + H2(g) 3g Na × 1mole Na = 0.1305 mole Na × 1H 2 =0.0652 mole H2 22.99g 2Na × 2.016 g = 0.1315 grams H2 = 0.13 grams of H2 1 mole H 2 3. ______________ What mass of silver will be precipitated when 7.32g of zinc reacts completely with silver nitrate? Zn(s) + 2 AgNO3(aq) → Zn(NO3)2(aq) + 2 Ag(s) 7.32 g Zn × 1moleZn = 0.1119 mole Zn 65.41g × 2 Ag =0.2238 mole Ag 1Zn × 107.9 g =24.1501 = 24.2 grams of Ag 1mole Ag 4. ______________ Potassium metal reacts with hydrochloric acid to produce aqueous potassium chloride and hydrogen gas. How many grams of potassium are required to produce 5.00 g of hydrogen gas? 2K + 2HCl à 2KCl + H2 5.00g H2 × 1mole H 2 =2.4802 mole H2 2.016g × 2K =4.9603 mole K 1H 2 × 39.1 g = 193.4524 = 193 grams K 1 mole K 5. ______________ Ethanol burns according to the following equation. If 655 g of water is produced, what mass of ethanol is burned? C2H5OH(aq) + 3O2(g) → 2CO2(g) + 3H2O(g) 46.068 g 655 g H2O × 1mole H 2O = 36.3566 mole H2O × 1C 2 H 5OH =12.1189 mole C2H5OH × = 558.2915 = 558 g of ethanol 18.016g 1moleC 2 H 5OH 3H 2O 6. ______________ How many grams of potassium chlorate, KClO3, must be decomposed to produce oxygen and 71.8 g of potassium chloride? 2KClO3 à 2KCl + 3O2 71.8g KCl × 1moleKCl = 0.9631 mole KCl 74.55g × 2KCl =0.9631 mole KClO3 2KClO3 × 122.55 g = 118.0294 = 118 grams KClO3 1 mole KClO3 [B] Limiting Reactant Problems 1. Calcium hydroxide, used to neutralize acid spills, reacts with hydrochloric acid according to the following equation: Ca(OH)2 + 2HCl àCaCl2 + 2H2O a. ______________ If you have spilled 6.3 mol of HCl and put 2.8 mol of Ca(OH)2 on it, which substance is the limiting reactant? 6.3 mole HCl × 1Ca(OH)2 = 3.15 mole of Ca(OH)2 needed. You have only 2.8 mole which isn’t enough. Ca(OH)2 is the limiting reactant. 2HCl b. ______________ How many moles of the excess reactant remain? Use the limiting reactant to do the calculations 2.8 mole Ca(OH)2 × 2HCl =5.6 mole of HCl needed. You have 6.3 (this also confirms that the HCl is in excess)…6.3 – 5.8 actually used 1Ca(OH)2 = 0.7 mole of HCl remaining Homework: Calculating with Chemical Equations 3 1/12/15 2. Aluminum oxidizes according to the following equation: 4Al + 3O2 à 2Al2O3 a. ______________ Powdered Al (0.048 mol) is placed into a container containing 0.030 mol O2. What is limiting reactant? 0.048 mole Al × 3O2 = 0.036 mole of O2 needed. You only have 0.030, so the O2 is the limiting reactant (there isn’t enough for this much Al) 4 Al b. ______________ How many moles of the excess reactant remain? Use limiting reactant to get the amounts actually used 0.030 O2 × 4 Al =0.04 mole of Al actually needed. 0.048 available – 0.040 needed = 0.008 mole of Al remaining. 3O2 [C] Percent Yield Problems 1. ______________ Dichlorine monoxide, Cl2O is sometimes used as a powerful chlorinating agent in research. It can be produced by passing chlorine gas over heated mercury (II) oxide according to the following equation. What is the percent yield, if the quantity of the reactants is sufficient to produce 0.86g of Cl2O but only 0.71 g is obtained? HgO + Cl2 àHgCl2 + Cl2O This is the simplest form of a percent yield problem because it already tells you much was actually produced and how much could have been produced. It’s basically a comparison of these two values. Actual gramamount 0.71 g ×100 à ×100 =82.5581=83%. This means that they obtained 83% of the ideal or expected mass. Predicted or Theoretical gram 0.86 g 2. In the commercial production of the element arsenic, arsenic(III) oxide is heated with carbon, which reduces the oxide to the metal according to the following equation: 2As2O3 + 3C à3CO2 + 4As a. ______________ If 8.87g of As2O3 is used in the reaction and 5.33 g of As is produced, what is the percent yield? This is a harder version because you have to first calculate how much should have been produced. In the previous problem, they just told you that 0.86 was expected. Here you have to do a whole stoichiometry problem to get to the expected value. 8.87 As2O3 × 1mole As 2O3 =0.0448 mole reacted × 4 As = 0.0897 mole of As expected × 74.92 g =6.718 = 6.72 grams, ideally 197.84 g 2 As 2O3 1mole As Now it’s like the first problem: 5.33 g ×100 =79.3396 = 79% yield 6.72g b. ______________ If 67 g of carbon is used up in a different reaction and 425g of As is produced, calculate the percent yield of this reaction. 67g C × 1moleC = 5.5787 mole C 12.01g 425 g ×100 =76.2642 = 76.3% 557.273 g × 4 As =7.4382 mole As 3C × 74.92 g =557.2734 grams of As, theoretically 1mole As 3. ______________ Huge quantities of sulfur dioxide are produced from zinc sulfide by means of the following reaction. If the typical yield is 86.78%, how much SO2 should be expected if 4897g of ZnS are used? 2ZnS(s) + 3O2(g) à2ZnO(s) + 2SO2(g) In this problem they tell you what the percent yield is and how much you started with. You have to figure out, using this starting gram amount, how much could be made (as if it were 100% yield) and then take 86.78% of this ideal amount. 4897 g ZnS × 1moleZnS =50.2359 moles ZnS × 2SO 2 = 50.2359 moles SO2 expected × 64.07 g = 3218.617 grams of SO2 expected 97.48 g 2ZnS 1moleSO 2 86.78% × 3118.617 =2793.1159=2793 grams if SO2, if the yield is only 86.78% xg ×100 =86.78% à x= 100 3218.617g 4. ______________ A process by which zirconium metal can be produced from the mineral zirconium (IV) orthosilicate, ZrSiO4, starts by reacting it with chlorine gas to form zirconium (IV) chloride. What mass of ZrCl4 can be produced if 862g of ZrSiO4 and 950.g of Cl2 are available? (You must first determine limiting reactant). ZrSiO4 + 2Cl2 à ZrCl4 +SiO2 + O2 862 g ZrSiO4 1moleZrSiO4 =4.7024 mole of ZrSiO4 present × 2Cl 2 =9.4048 mole Cl2 needed × 70.9 g =666.8027 g Cl2 needed. We 183.31g 1ZrSiO4 1moleCl 2 have way too much (actually have 950; so Cl2 is in excess); You can use the ZrSiO4 value to predict how much ZrCl4 will be produced. 4.7024 mole of ZrSiO4 present × 1Zr Cl 4 =4.7024 mole of ZrCl4 will be produced, ideally × 233.02g =1095.7532=1010. g or 1.10x103 g 1ZrSiO4 1moleZrCl 4 Homework: Calculating with Chemical Equations 4 1/12/15