Solutions Level II Section I: Reading Solubility Curves – Use the
advertisement

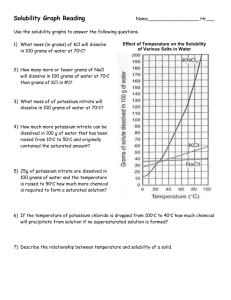
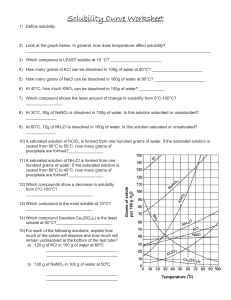
Solutions Level II Section I: Reading Solubility Curves – Use the solubility curve below to answer the following questions. 1. What relationship exists between solubility and temperature for the ionic substances shown? 2. What relationship exists between solubility and temperature for the only gas, SO2, on the graph? Indicate if the following solutions are saturated, unsaturated or supersaturated. 3. 20 grams of NH3 completely dissolved in 100 grams of water at 80ºC. 4. 80 grams of KNO3 completely dissolved in 100 grams of water at 55ºC? 5. 112 grams of NaNO3 completely dissolved in 100 grams water at 45 ºC? 6. 10 grams of KClO3 completely dissolved in 100 grams water at 60ºC? 7. 30g KCl in 100g water at 10ºC? Section II: Electrolytes - Complete the following table. The first one has been done for you as an example. Sodium chloride Formula? Ionic, Acid/Base, or Covalent? Soluble in water? Electrolyte? NaCl Ionic Yes Yes 1. Acetic Acid 2. Silver bromide 3. Hydrofluoric acid 4. Carbon dioxide 5. Strontium hydroxide 6. Magnesium chlorate 7. Ammonium nitrate Section I: 1.Directly related; 2. Inversely related; 3. SS 4. U 5.S 6. U 7.S Section II: 1.Y 2. N 3.Y 4.N 5. Y 6.Y 7.Y Section III: Determining Concentration – In order to use a solubility curve, you must be able to convert your units to the units the curve are in. The curve above is measured in grams solute/100g H2O, so we must convert our units to grams solute/100g H2O. Be sure to use correct sig figs! No Work = No Credit = No Kidding! Example: 60.0 grams KClO3 dissolved in 300.0 g water 60.0/300.0g = 0.2 g KClO3/g water 0.2 g KClO3/g water x 100 = 20g KClO3/100 g water 1. 90.0g KNO3 dissolved in 250g water 3. 25g SO2 dissolved in 50.0g water 2. 45.5g NaCl dissolved in 150g water 4. 215g KNO3 dissolved in 500g water Section IV: Molarity Calculations – In each of the following problems, determine the molarity of the solution described. Use the following example to help you. No Work = No Credit = No Kidding! Example: What is the molarity of a solution in which 15.5 grams of NaCl is dissolved into 125 mL of solution? Step 1: Convert grams of NaCl to moles 15.5g x 1 mol NaCl = 0.265 mol 58.443 g NaCl Step 2: Convert mL of solution to L 125 mL x 1L = 0.125L 1000mL Step 3: Divide g/L 0.265g/0.125L = 2.12 g/L 1. 150g NaCl dissolved in 1L of water 4. 125g KClO3 in 255mL water 2. 3.5 moles KNO3 dissolved in 2.5L water 5. 45 g KCl in 0.5L water 3. 450g NH3 dissolved in 150mL solvent 6. 35g NaCl dissolved in 0.5L water Section V – Dilutions In some cases, it is necessary to lower the concentration of a solution. This process is known as dilution. The equation used in diultions is V1M1 = V2M2, where V is volume and M is the molarity of the solution. Use the following example to help you answer the questions that follow. No Work = No Credit = No Kidding! Example: 500. mL of a 2.5 molar solution needs to be diluted to 1.5 molar concentration. How much water should be added to reach this concentration? Solving for: V2 V2 = V1M1/M2 V2 = (500mL x 2.5M)/(1.5M) = 833mL We already have 500mL, so we need to add 333mL! 1. 750. mL of a 5 molar solution needs to be diluted to 1M. How much water should be added to reach this concentration? 2. If 30.0 mL of 12.0M hydrochloric acid is diluted to a volume of 500 mL, what is the molarity of the diluted solution? 3. If, when water is added to 275 mL of 16.0M nitric acid solution, the new molarity was found to be 1.35M, how many mL of water were added to the original solution? Section III: 1. 36g KNO3/100g H2O 2. 30g NaCl/100g H2O 3. 50g SO2/100g H2O 4. 40g KNO3/100g H2O Section IV: 1. 2.6 mol/L 2. 1.4 mol/L 3. 150 mol/L 4. 4.00 mol/L 5. 1.2 mol/L 6. 1.2 mol/L Section V: 1. 2000mL 2. 0.7M 3. 2980 mL