Chapter 17: Electron Transport and Oxidative
advertisement

Chapter-17
1
Takusagawa’s Note©
Chapter 17: Electron Transport and Oxidative
Phosphorylation
-
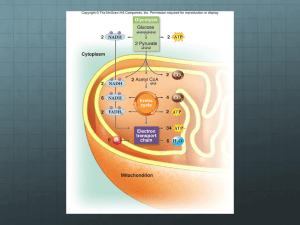
The complete oxidation of glucose by molecular oxygen is:
C6H12O6 + 6O2 → 6CO2 + 6H2O
ΔG°′ = -2823 kJ/mol
- This equation is broken down into two half-reactions.
1. The glucose carbon atoms are oxidized by water molecules:
C6H12O6 + 6H2O → 6CO2 + 24H+ + 24e{Glycolysis and citric acid cycle}
2. The molecular oxygen is reduced by the protons and electrons produced by glucose
oxidation:
{Electron transport and oxidation}
6O2 + 24H+ + 24e- → 12H2O
- The oxidation of glucose carbon atoms is carried out in glycolysis and the citric acid cycle,
and the produced protons and electrons are stored in NADH and FADH2 molecules.
1
-
-
Takusagawa’s Note©
2
Chapter-17
The electrons from NADH and FADH2 are injected into the electron-transport chain in the
inner membrane of mitochondrion.
When the electron passes through the electron-transport chain into the O2 reduction chamber
(a protein complex), the protons in the mitochondrial matrix are expelled to the
intermembrane space so as to generate the pH gradient between the matrix and the
intermembrane space of a mitochondrion.
The free energy stored in the resulting pH gradient drives the synthesis of ATP from ADP
and Pi.
This process is called oxidative phosphorylation.
1. THE MITOCHONDRION
Outer membrane
Cristate
Matrix
Inner membrane
-
is located inside of cells.
Size: Ellipsoid of ~0.5 μm diameter and ~1 μm length.
Proteins that mediate electron transport and oxidative phosphorylation are bound to the inner
membrane.
Inner compartment called matrix contains:
- soluble enzymes for oxidative metabolism.
- substrates
- nucleotide cofactors
- inorganic salts
- DNA, RNA, ribosomes
Outer membrane:
- has porins which have non-specific pores that permit free diffusion up to 10 kD molecules.
Thus the concentration of ions in the intermembrane space and cytosol are nearly the same.
Inner membrane:
- is ~75% protein.
- is impermeable to most hydrophilic substances, freely permeable only to O2, CO2 and H2O.
- contains respiratory chain proteins and transport proteins that control [ATP], [ADP],
[pyruvate], [Ca2+], [Pi], and etc.
- generates ionic gradients between cytosol and mitochondria.
2
Chapter-17
3
Takusagawa’s Note©
Inner membrane
Cytosol
||
Matrix
cytosolic NADH →
||
← oxaloacetate, Acetyl-CoA
||
← precursors of glucose &
||
fatty acid biosynthesis
ADP & Pi →
||
← ATP produced in mitochondrion.
Transports in the intermembrane
Pi transport
- Pi is transported via Pi-H+ symport driven by ΔpH.
Ca2+ transport
- Ca2+ is a second messenger, thus [Ca2+] must be precisely controlled.
- Mitochondrion, endoplasmic reticulum and extracellular spaces act as Ca2+ storage tanks.
- Influx (intermembrane space → matrix) and efflux (matrix → intermembrane space) are
separately mediated.
- Influx is a uniport driven by membrane electro-potential (ΔΨ, negative inside) generated by the
electron-transport chain.
- Efflux is an antiport driven by Na+ gradient across the inner membrane.
Symport
Antiport
Uniport
Ca2+ signal transduction in muscle
[Ca2+] in cytosol of a muscle cell is increased by muscle activity → [Ca2+]↑ in matrix → activate
enzymes of citric acid cycle → [NADH]↑ → generate ATP which provides muscle activity.
3
Chapter-17
4
Takusagawa’s Note©
NADH transport (across the inner mitochandrial membrane)
- Cytoplasmic shuttle systems “transport” NADH across the inner mitochondria membrane
- Cytosol NADH generated by glycolysis cannot pass through the inner mitochondrial
membrane.
- Thus electrons (2e-) of cytosolic NADH are transported into the mitochondrial matrix.
- There are two NADH shuttles.
Glycerophosphate shuttle (Insects)
1. The protons and electrons of NADH are transferred to dihydroxyacetone phosphate.
2. The reduced product, 3-phosphoglycerol transfers the protons and electrons to a FAD in
flavoprotein dehydrogenase located in the inner mitochondrial membrane.
3. The electrons are injected into the electron-transport chain in the inner mitochondrial
membrane.
-
Glycerophosphate shuttle generates 2ATP for every cytoplasmic NADH reoxidized.
4
Chapter-17
5
Takusagawa’s Note©
Malate-aspartate shuttle
- is the mammalian system.
- is more energy efficient than glycerophosphate shuttle.
1. The cytosolic NADH reduces oxaloacetate to malate.
2. The malate is transported into the matrix through a malate-α-ketoglutarate carrier (antiport).
3. The transported malate reduces the NAD+ in the matrix to NADH, and becomes
oxaloacetate.
4. The oxaloacetate receives an amino group from a Glu, and becomes Asp. The deaminated
Glu becomes an α-ketoglutarate.
5. Asp in the matrix is transported into the cytosol through a glutamate-aspartate carrier
(antiport).
6. The transported Asp donates its amino group to α-ketoglutarate, and Asp becomes
oxaloacetate, and α-ketoglutarate becomes Glu.
-
Malate-aspartate shuttle yields 3ATP for every cytosolic NADH, one more than the
glycerophosphate shuttle.
5
Chapter-17
Takusagawa’s Note©
6
2. ELECTRON TRANSPORT
A. Thermodynamics of electron transfer
Electrons are transported from NADH and FADH2 to O2.
The free energy (G°′) and the reduction potential (E°′) under the standard biochemical
conditions are defined as the reaction of 1 M reactants and products in an aqueous solution
(pH 7).
Standard reduction potential difference, ΔE°′ is: ΔE °′ = E °′(e acceptor) - E °′(e donor)
Example of ½O2 reduction by NADH.
- The reduction potentials of NAD+ and ½O2 are: (see Table 15-4)
NAD+ + H+ + 2e- ↔ NADH
E °′ = -0.315 V --- (1)
½O2 + 2H+ + 2e- ↔ H2O
E °′ = 0.815 V --- (2)
-
-
Overall reaction of ½O2 reduction by NADH is:
½O2 + NADH + H+ ↔ H2O + NAD+
The above reaction is the difference between the equations (2) and (1), i.e., (2) - (1)
½O2 + 2H+ + 2e- ↔ H2O
E °′ = +0.815 V
+
+
-) NAD + H + 2e ↔ NADH
E °′ = -0.315 V
⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯
½O2 + NADH + H+ ↔ H2O + NAD+
ΔE °′ = 0.815 - (-0.315) = 1.130 V
In the above reaction,
O2 receives electrons
E °′(e acceptor)
NADH donors electrons
E °′(e donor)
ΔE °′ = 0.815 - (-0.315) = 1.130 V
= +0.815 V
= -0.315 V
The relation between the standard free energy change ΔG°′ and the standard reduction potential
change ΔE°′ is: ΔG°′ = -nFΔE °′
where:
F= 96494 C·mol-1 of electrons (Faraday constant).
n = number of electron transferred per one mole of reactant.
1 V = 1 J·C -1
Therefore, the standard free energy change of the NADH oxidation by ½O2 reduction is:
mol e −
C
× 96494
× 1130
.
J ⋅ C -1
ΔG°′ = −2
mol reactant
mol e
= -218 kJ·mol-1
-
Oxidation of NADH by O2 (2e- transfer) under standard biochemical condition releases 218
kJ of free energy.
An ATP synthesis (ADP + Pi → ATP) requires 30.5 kJ/mol free energy.
Thus, several moles of ATP can be synthesized from 218 kJ of free energy of (½O2 + NADH →
NAD+ + H2O) reaction.
Actually, 218 kJ free energy is broken up into three packets.
6
-
Takusagawa’s Note©
7
Chapter-17
Each of packets is coupled with one ATP synthesis.
Efficiency of the ATP synthesis is: 100 × (3 × 30.5)/218 = 42% at standard biochemical
condition.
Efficiency at physiological condition ≈ 70%.
(Automobile engine efficiency < 30%)
B. Sequence of electron transport
- The electrons of NADH are injected into the Complex I which is a transmembrane multisubunit protein complex.
- The electrons of FADH2 are transferred into the Complex II.
- Those electrons are carried by a CoQ (coenzyme Q (ubiquinone)) to the Complex III.
- The electrons are further transferred to the Complex IV (O2 reduction chamber) by
cytochrome c (peripheral membrane protein).
- When the electron passes through each Complex (Complex I, III and IV), an amount of H+ is
pumped out from the mitochondrial matrix to the inner membrane space, which can produce
one mole of ATP.
- Protein complexes (I, III and IV) catalyze the following reactions.
Complex I
NADH + CoQ(oxidized) → NAD+ + CoQ(reduced)
ΔE°′ = 0.360V
ΔG°′ = -69.5 kJ/mol
(One ATP)
Complex II
FADH2 + CoQ(ox) → FAD + CoQ(red)
ΔE°′ = 0.015V ΔG°′ = -2.9 kJ/mol
(Zero ATP)
↓
Complex III
CoQ(red) + cytochrome c(ox) → CoQ(ox) + cytochrome c(red)
ΔE°′ = 0.190V ΔG°′ = -36.7 kJ/mol
(One ATP)
↓
Complex IV
Cytochrome c(red) + ½O2 → cytochrome c(ox) + H2O
ΔE°′ = 0.580V ΔG°′ = -112 kJ/mol
(One ATP)
Intermembrane
space space
+
+
H
H
+
e
Q
I
e
H
Cyto c
-
e
-
-
IV
III
e
NADH
Matrix
-
II
NAD
+
e
Succinate
-
O2
Fumarate
7
H2O
Takusagawa’s Note©
8
Chapter-17
Mitochondrial electron transport chain with reduction potentials
Electron-transport chain has been elucidated through the use of inhibitors
- The O2 consumption of mitochondria can be measured by an oxygen electrode, i.e., by
measuring the electric currency with a volt meter.
e
-
e
Mitochondrion
-
O2
Bubble
O2
H2
+
H
H2O
Electrolysis
Bio-cell
8
Takusagawa’s Note©
9
Chapter-17
The following compounds can inhibit the specific Complex’s activities.
- Rotenone and amytal inhibit the Complex I activity.
- Antimycin A inhibits the Complex III activity.
- CN- ion inhibits the Complex IV activity.
- Structures are listed in the next page.
The following reducing agents can inject electrons into the specific Complexes.
- β-Hydroxybutyrate reduces NAD+ to NADH which injects electrons into Complex I.
- Succinate reduces FAD to FADH2 which injects electrons into Complex II.
- Reduction of tetramethyl-p-phenylenediamine (TMPD) by ascorbic acid produces
electrons which are transferred to cytochrome c.
OH
1.
O
H3C CHCH2 COO
β-hydroxybutyrate
-
H3C
CH2 COO
acetoacetate
-
+
NAD
+
NADH + H
+
NAD
-
e
3.
Succinate
Complex I
FAD
-
FADH2
e
Complex II
2. Rotenone or Amytal
-
e
Fumarate
Complex III
5.
Tetramethyl-p-phenylendiamine
(TMPD)
4. Antimycin A
-
e
cytochrome c
e
-
e
+
Ascorbic acid
Complex IV
6. CN-
[O2] is reduced
Summary of experiment
1. Add β-hydroxybutyrate into the reaction cell → O2 consumption is increased.
2. Add rotenone or amytal into the reaction cell → O2 consumption is stopped (Complex I is
inhibited).
3. Add succinate into the reaction cell → O2 consumption is resumed.
4. Add antimycin A into the reaction cell → O2 consumption is stopped (Complex III is inhibited).
5. Add TMPD + ascorbic acid into the reaction cell → O2 consumption is resumed.
6. Add CN- into the reaction cell → O2 consumption is stopped (Complex IV is inhibited).
9
Chapter-17
10
Structures of Inhibitors and Reducing Agents
10
Takusagawa’s Note©
Chapter-17
11
Takusagawa’s Note©
Phosphorylation and oxidation are coupled events
- O2 consumption is minimal without ADP and Pi in the reaction cell, indicating that the
oxidation (O2 → H2O) and the phosphorylation (ADP + Pi → ATP) are coupled events.
- By measuring the O2 consumption and the ATP synthesis, we can determine the number of
ATP molecules per specific electron carrier molecules (NADH, succinate and ascorbate).
P/O ratio
- is the ration of ATP synthesis per ½O2 (= O) consumption.
- One NADH (½O2 consumption) produces three ATPs → P/O = 3
- One succinate (½O2 consumption) produces two ATPs → P/O = 2
- One ascorbate (½O2 consumption) produces one ATP → P/O = 1
Experimental procedure:
- The reaction cell contains mitochindria and all necessary materials for the oxidative
phosphorylation except for ADP. Thus the reaction cell does not consume O2.
1. 90 μM of ADP and excess of β-hydroxybutyrate is added in the reaction cell.
15 μM of O2 is consumed.
NADH + ½O2 + xADP + xPi → NAD+ + H2O + xATP
(30)NADH + (15)O2 + (90)ADP + (90)Pi → (30)NAD+ + (30)H2O + (90)ATP
(15)O2 = (30)O, thus
x = 90/(30) = 3
2. The Complex I’s inhibitor, rotenone, is added to stop the Complex I’s activity, and then 90
μM of ADP and excess of succinate is added into the reaction cell.
22.5 μM of O2 is consumed.
FADH2 + ½O2 + yADP + yPi → FAD + H2O + yATP
(45)FADH2 + (22.5)O2 + (90)ADP + (90)Pi → (45)FAD + (45)H2O + (90)ATP
(22.5)O2 = (45)O, thus
y = 90/(45) = 2
3. The Complex III’s inhibitor, antimycin A, is added to stop the Complex III’s activity, and
then 90 μM of ADP and excess of ascorbate/TMPD is added into the reaction cell.
45 μM of O2 is consumed.
Ascorbate + ½O2 + zADP + zPi → Dehydroascorbate + H2O + zATP
(90)Ascorbate + (45)O2 + (90)ADP + (90)Pi → (90)Dehydroascorbate + (90)H2O + (90)ATP
(45)O2 = (90)O, thus
z = 90/(90) = 1
-
These P/O ratio numbers indicate that one ATP is synthesized when the two electrons pass
through every Complex I, Complex III, and Complex IV.
11
Chapter-17
12
Takusagawa’s Note©
C. Components of the Electron-Transport Chain
- The Complexes contain various prosthetic groups (FMN, FAD, heme, and Fe-S).
2H+
12
-
Takusagawa’s Note©
13
Chapter-17
1. Structure and function of Complex I
Name: NADH-Coenzyme Q reductase.
receives electrons from NADH, passes those to FMN and to various Fe-S clusters, and then
to CoQ.
probably the largest protein component in mitochondria (850 kD).
contains 6 to 7 Fe-S clusters.
Three types of Fe-S clusters
S
Cys
S Cys
S
Fe
Cys
S
S Cys
Cys
Cys
S
Fe
S
[Fe-S]
S
S
Fe
S Cys
S
[2Fe-2S]
S=S
-
Cys
S
S
Fe
Fe
S
Fe
S
S
Cys
S
Fe
Cys
S
[4Fe-4S]
2-
[2Fe-2S] and [4Fe-4S] are the most common types.
Oxidation states of irons are between +2 and +3.
For example,
Ferredoxin is an electron carrier protein and contains a [4Fe-4S] cluster. The oxidation state
of irons are:
Oxidized form: one Fe(II) and three Fe(III)
Reduced form: two Fe(II) and two Fe(III)
Note: [4Fe-4S] cluster carries only one electron at one time.
2e- carriers: NADH
CoQ
FADH2
FMNH2
1e- carriers:
-
Cys
Cys
Cytochromes
Fe-S clusters
contains one flavin mononucleotide (FMN) which is a redox-active prosthetic group.
FMN differs from FAD only by the absence of the AMP group.
Redox states of FMN and Coenzyme Q (CoQ) are shown next page.
Both coenzymes form stable semiquinone free radical states.
13
Chapter-17
14
Takusagawa’s Note©
2. Complex II
- Name: Succinate-Coenzyme Q Reductase.
- passes electrons from succinate to CoQ.
- contains succinate dehydrogenase & three hydrophobic subunit.
- contains covalently bound FAD, one [4Fe-4S], one [2Fe-2S], one cytochrome b560.
- The bound FAD is reduced to FADH2 in the citric acid cycle by the oxidation of succinate to
fumarate.
3. Complex III
- Name: Coenzyme Q-Cytochrome c Reductase.
- passes electron from reduced CoQ to cytochrome c.
- contains two b-cytochromes (bL (566 nm) and bH (562 nm)), one cytochrome c1, and one
[2Fe-2S] cluster.
14
Chapter-17
15
Takusagawa’s Note©
Cytochromes
- are electron-transport heme proteins.
- Iron in heme reversibly alternate between Fe(II) and Fe(III) oxidation states during electron
transport.
- visible absorption spectra of reduced forms have three characteristic peaks (α, β, γ) between
400 and 600 nm.
- The mitochondrial membrane contains three cytochrome species, cytochrome a, b, and c.
- The spectrum of beef heart mitochondrial membrane indicates that the α-bands of
cytochrome a, b and c are around 600 nm, 560 nm and 550 nm, respectively (seen in Fig. 2016 (b)).
-
Within each group of cytochromes, the absorbing maxim of α bands are slightly different by
different heme group environments.
Absorbing maximum of α bands are used to nomenclature.
e.g., cytochrome b566 has the α band at 566 nm.
15
Chapter-17
16
Takusagawa’s Note©
Cytochrome a, b and c contain different porphyrin a, b, and c, respectively
The hemes of cytochrome a and b are quite similar, but the heme of cytochrome c is different
The heme of cytochrome c is covalently attached to the protein and His and Met are
coordinated to the iron, whereas the hemes of cytochrome a and b are coordinated by two His
residues, and are not covalently attached to the proteins.
16
Chapter-17
17
Takusagawa’s Note©
Cytochrome c
- is a peripheral membrane protein and contains a c-type heme.
- alternately binds to cytochrome c1 of Complex III and cytochrome c oxidase (Complex IV).
- Its function is to shuttle electrons between complex III and IV.
- carries one electron from Complex III to Complex IV.
Structure of cytochrome c
Cyto C
17
Takusagawa’s Note©
18
Chapter-17
4. Cytochrome c oxidase (Complex IV)
- catalyzes one-electron oxidations by four consecutive reduced cytochrome c molecules and
concomitant four-electron reduction of one O2 molecule:
4(Cytochrome c2+) + 4H+ + O2 → 4(Cytochrome c3+) + 2H2O
- contains two a-type hemes (a and a3) that alternate between Fe2+ and Fe3+ oxidation states.
- contains two Cu atoms that alternate between Cu+ and Cu2+.
- An electron carried by a cytochrome c is received at the CuA cluster (2Cu-2S complex), then
transferred to cytochrome a, and then the cytochrome a3-CuB binuclear complex where the
molecular oxygen is reduced to water molecule.
Cytochrome c
N
N
Fe S
Met
CH3
His
-
e
N
Cys
N
S
O C
Cu
C O
Cytochrome c oxidase
Cu
S
CuA
N
Cys
N
-
e
N
N Fe N
N
Cytochrome a
CuB
-
e
N
N
N
N Fe
Cu N
O O
N
N
Cytochrome a3
N
Molecular oxygen (O2)
18
Chapter-17
19
Takusagawa’s Note©
Reduction sequence for the reduction of O2 by cytochrome c oxidase
1. The binuclear Fe(III)a3-Cu(II)B complex receives one electron and becomes Fe(III)-Cu(I).
2. The Fe(III)-Cu(I) receives the second one electron and becomes Fe(II)-Cu(I).
3. O2 binds between the Fe(II) and Cu(I), Fe(II)-O-O-Cu(I).
4. Internal electron redistribution causes the Fe(III)-O--O--Cu(II) stable peroxy adduct.
5. A further one-electron transfer and a proton acquisition cause Fe(III)-O--OH Cu(I).
6. A further proton acquisition cause Fe(IV)=O2- H2O-Cu(II).
7. The fourth one-electron transfer together with a proton rearrangement yields Fe(III)-OHHO-Cu(II).
8. Two more proton acquisition releases 2H2O and the binuclear complex is back to
Fe(III)a3-Cu(II)B.
Note: O2 binds to Fe2+. In Hb and Mb, O2 also binds to Fe2+.
19
Chapter-17
20
Takusagawa’s Note©
3. OXIDATIVE PHOSPHORYLATION
A. Chemiosmotic hypothesis by Peter Mitchell (1961).
- The free energy of electron transport is conserved by pumping H+ from the mitochondrial
matrix to the intermembrane space so as to create an electrochemical H+ gradient across the
inner mitochondrial membrane. The electrochemical potential of this gradient is harnessed
to synthesize ATP.
There are 4 key observations that support the chemiosmotic hypothesis.
1. Oxidative phosphorylation requires an intact inner mitochondrial membrane.
2. The inner membrane is impermeable to H+, OH-, K+, Cl-.
3. There is a measurable electrochemical gradient across the inner membrane.
4. “Uncouple” compounds that increase the permeability of the inner membrane to protons do
not disrupt the electron transport (O2 → H2O), but inhibit the ATP synthesis.
B. Proton gradient generation
Proton pumping is an endergonic process
- Let us consider a case that the protons in the mitochondrial matrix ([H+]in) are pumped out to
the intermembrane space ([H+]out) against the proton gradient. The free energy change is:
[H + ]out
ΔG = RT ln +
+ ZF(Ψout − Ψin )
Ψout = positive; Ψin = negative (see Fig. 20-23).
[H ]in
ΔG = RT×2.3(log[H+]out - log[H+]in) + ZFΔΨ
ΔG = 2.3RT[pH(in) - pH(out)] + ZFΔΨ
where Z = charge on the proton (+1), F = Faraday constant (96494 C/mol), ΔΨ = membrane
potential (when an ion is transported from negative to positive, ΔΨ is positive).
- ZFΔΨ > 0 and pH(in) > pH(out), thus ΔG > 0 (endergonic)
- Example of liver mitochondrion:
ΔΨ = 0.168 V across 80 Å (Note: this corresponds ~210 kV across 1cm)
ΔpH = 0.75
ΔG = 2.3 × 8.3145J·K-1mol-1 × 310K × 0.75 +1 × 96494J·V-1·mol-1 × 0.168V
= 20657.17 J·mol-1 = 20.7 kJ·mol-1
20
Takusagawa’s Note©
21
Chapter-17
Redox loop mechanism
Proton pump mechanism of proton transport
- The transfer of electrons results in conformational changes in the complex structures. These
conformational changes translocate the protons from the mitochondrial matrix to the
intermembrane space.
Matrix
+
H
Innermembrane
O H
O H
Proton transfer
IMS
O H
H O
H O
H O
Conformational change
21
+
H
+
H
O H
O H
O H
2nd proton transfer
Chapter-17
22
Takusagawa’s Note©
C. Mechanism of ATP synthesis.
- ATP is synthesized by proton-translocating ATP synthase (proton pumping ATPase,
F1F0-ATPase).
- It is located in the inner mitochondrial membrane.
-
Proton-translocating ATP synthase is a multi-subunit transmembrane protein.
It has two major substructures, i.e., F1 and F0.
F1 --- Water soluble peripheral membrane protein composed of 9 subunits, α3β3γδε.
F0 --- Water insoluble transmembrane protein composed of 10~12 subunits.
Stalk --- between F1 and F0 which contains oligomycin-sensitivity-cofactorring protein
(OSCP) and coupling factor 6 (F6).
22
Chapter-17
Takusagawa’s Note©
23
The catalytic sites of ATP synthesis is located between the α and β subunits of F1.
- F1 itself cannot synthesize ATP, but can hydrolyze ATP by reverse reaction.
-
-
Oligomycin (antibiotic) inhibits ATP synthesis by binding to a subunit of F0 (not OSCP) so
as to interfere with H+ transport through F0.
Dicyclohexylcarbodiimide (DCCD) also inhibits proton transport through F0 by reacting the
carboxyl group of Glu (or Asp in E. coli) that is located in a lipid environment (buried in the
membrane), indicating that the Glu is involved in the proton transport.
Mammalian F0 contains six copies of DCCD-binding proteins.
-
O
Glu
O
X-ray structure of F1 reveals:
- F1 has pseudo-six fold symmetry, i.e., α and β subunits have nearly identical folds and are
arranged alternately.
- The (αβ)3 are held by a 90 Å α helix of γ-subunit that is located near the center of the (αβ)3.
- The three αβ dimers fold slightly different to each other.
γ-Subunit
23
Takusagawa’s Note©
24
Chapter-17
Proton-translocating ATP synthase is driven by conformational changes in F1
- Conformational changes might be driven by the rotation of the γ subunit in the catalytic
assembly, (αβ)3.
- The (αβ)3 has three binding states:
- Tightly binding state (T); Loosely binding state (L); Open binding state (O)
- ATP synthesis pathway is:
1. ADP + Pi bind to the “loosely binding site (L).
2. A negative free energy is produced by the proton flow in the ATP synthase from the
intermembrane space to matrix. This free-energy (E) changes the conformation of (αβ)3 of
F1 to: T→ O, L → T and O → L.
3. ATP is synthesized at the T site while the ATP in the O site dissociates.
A cartoon representation of the oxidative phosphorylation
Intermembrane space
+
+
H
+
H
e
H
-
+
+
+
H H H
(I)
Motor
e
Proton
pump
-
(III)
Motor
(IV)
Proton
pump
Motor
e
eNADH NAD +
(V)
+
4H + O2
Matrix
24
-
2H2O
Proton
pump
Synthesizer
ADP
+
Pi
ATP
Chapter-17
25
Takusagawa’s Note©
D. Uncoupling of oxidative phosphorylation
Important remark:
- The “tight coupling” between electron transport (oxidation of NADH and FADH2 by O2) and
phosphorylation (ATP synthesis) in the mitochondrion depends on the impermeability of the
inner mitochondrial membrane.
Compounds that disrupt the “tight coupling” are called “uncoupling agents”.
- 2,4-dinitrophenol (DNP) and carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP)
are “uncoupling agents”, and also proton-transporting ionophores.
- These molecules are lipophilic weak acids which can diffuse easily through the
mitochondrial membrane.
- These molecules carry the H+ in the intermembrane space to the matrix. Thus, the proton
gradient created by the electron transport is reduced. Consequently the ATP synthesis is
slowed down, but not the electron transport.
25
Chapter-17
26
Takusagawa’s Note©
Natural uncoupling
Brown adipose tissues (brown fat) contain mitochondria, thus they are brown since cytochrome c
is red color.
- Note: white adipose tissues do not have mitochondrion.
- The mitochondria in brown fat contain uncoupling protein (UCP, called thermogenin).
- UCP is a transmembrane proton channel protein.
- When H+ passes through the UCP, heat is generated instead of ATP.
- This uncoupling system in brown fat is controlled by the hormone norepinephrine.
- The channel is:
Opened by norepinephrine signals: (cAMP→→→triacylglycerol lipase→free fatty acid).
Blocked by [ATP], [ADP], [GTP] and [GDP].
26
Takusagawa’s Note©
27
Chapter-17
4. CONTROL OF ATP PRODUCTION
An adult woman requires 6250~7500 kJ (6250 ~ 7500 kJ = 200 mol ATP to ADP + Pi) of
metabolic energy per day (200 mol of ATP = ~94 kg).
Total amount of ATP present in body is < 0.1 mol.
- Thus, ATP must be constantly produced.
However, consumption rate of ATP at sleeping and vigorous exercising times are 100-fold
different.
- Therefore, ATP productions are under strict coordinated control so that ATP is never
produced more rapidly than necessary.
Control mechanisms:
1. Glycolysis
2. Glycogenolysis (glycogen breakdown)
3. Citric acid cycle
4. Oxidative phosphorylation
⎫
⎬ have been discussed in previous chapters.
⎭
A. Control of Oxidative Phosphorylation
Pathway from NADH to cytochrome c functions near equilibrium (ΔG ≈ 0).
1/3(NADH) + cytochrome c3+ + ADP + Pi ↔ 1/3(NAD+) + cytochrome c2+ + ATP
Equilibrium constant is:
1
[
] [ ] [ ATP]
[ ] [ ADP] ⋅ [ P ]
⎛ NAD + ⎞ 3 c 2+
⎟⎟
Keq = ⎜⎜
3+
⎝ [ NADH ] ⎠ c
1.
-
[20.2]
i
Cytochrome c oxidase
Cytochrome c oxidase reaction is irreversible, therefore, it can be the control site.
Cytochrome c oxidase activity depends on the availability of the reduced cytochrome c2+.
Thus equation [20.2] is rewritten to:
[ ]
[c ]
c 2+
3+
1
⎛ [ NADH ]⎞ 3 ⎛ [ ADP] ⋅ [ Pi ] ⎞
⎟ ⎜
= ⎜⎜
⎟ K eq
+ ⎟
⎝ NAD ⎠ ⎝ [ ATP] ⎠
[
]
[ NADH] ratio and ATP mass action ratio [ ATP] .
[ ADP][ Pi ]
[NAD + ]
[ NADH] ↑ and ATP mass action ratio [ ATP] ↓, [cytochrome c 2+] ↑. In this case,
- When
[ ADP][ Pi ]
[NAD + ]
-
[cytochrome c 2+] is functions of
ATP synthesis is maximal.
-
Whereas, when
[ NADH] ↓ and ATP mass action ratio [ ATP] ↑, [cytochrome c 2+] ↓.
[ ADP][ Pi ]
[NAD + ]
In this case, ATP synthesis is minimal.
27
28
Chapter-17
2. ATP, ADP and Pi transport systems
- Mitochondrial innermembrane is
impermeable to ATP, ADP, Pi.
- Specific transport systems which
communicate between the two
compartments (matrix and cytosol) can
control the oxidative phosphorylation.
- Remember, the electron-transport
(oxidation by O2) and phosphorylation
(ATP synthesis) are coupled events.
Without enough ADP, the oxidative
phosphorylation is stopped in
mitochondria.
B. Coordinated control of ATP production
- ATP mass action ratio depends on the
[NADH]/[NAD+] ratio.
1
[ ]
] [ ]
3+
[ ATP] = ⎛⎜ [ NADH ]⎞⎟ 3 c K
[ ADP] ⋅ [ Pi ] ⎜⎝ NAD + ⎟⎠ c 2 + eq
[
-
Thus the activities of
phosphofructokinase (PFK) in glycolysis,
pyruvate dehydrogenase,
citrate synthase,
isocitrate dehydrogenase,
α-ketoglutarate dehydrogenase
in the citric acid cycle
are regulated by adenine nucleotides
(ATP: inhibitor; ADP & AMP: activators)
or NADH or both and by certain
metabolites, such as citrate.
Citrate inhibits glycolysis (i.e., inhibits PFK)
- Citric acid cycle is slowed down at
isocitrate dehydrogenase and αketoglutarate dehydrogenase by
[NADH]/[NAD+]↑ and [ATP] ↑.
- Thus, [Citrate] ↑. Therefore citrates can
leave the mitochondrion to cytosol, then
inhibits the PFK activity.
28
Takusagawa’s Note©
Chapter-17
29
Takusagawa’s Note©
C. Physiological implications of aerobic versus anaerobic metabolism
Anaerobic glycolysis:
C6H12O6 + 2ADP + 2Pi → 2(lactate) + 2H+ + 2H2O + 2ATP
Aerobic glycolysis:
C6H12O6 + 38ADP + 38Pi + 6O2 → 6CO2 + 44H2O + 38ATP
-
Thus, aerobic metabolism is 19 times more efficient than anaerobic metabolism (if the lactate
conversion is not taken into account).
By switching aerobic to anaerobic metabolism, ATP synthesis is drastically decreased.
But ATP is produced by anaerobic glycolysis much more rapidly than oxidative
phosphorylation (~100 times faster).
Cancer cell metabolism
Certain cancer cells produce more lactic acid under aerobic condition than do normal cells.
Because:
- The cancer cells produce pyruvate more rapidly than the citric acid cycle can accommodate
because the coordinated control system is broken down.
- The other explanation is that ATP consumption in the cancer cells is too fast to replenish
ATP through oxidative phosphorylation.
Cardiovascular disease
Undetectable heart attack (myocardial infarction) and stroke can be detected by measuring Htype lactate dehydrogenase in blood and/or intracellular pH. Why?
- Heart and brain tissues require constant O2 supply to produce constantly ATP. However, if
O2 supply is interrupted, ATP must be synthesized by anaerobic pathway. Thus [lactate] is
increased, and consequently lactate dehydrogenase is increased. Similarly the intracellular
pH is decreased (i.e., [H+] is increased).
-
Actually the following chain events are occurred in an undetectable heart attack:
1. [O2] in tissue is decreased since bloodstream is blocked.
2. [ATP] in tissue is decreased.
3. Na+-ATPase pump is stopped.
4. Cells swell and consequently break because the osmotic balance is broken.
5. Cellular proteins flow out and into bloodstream.
6. Thus, specific proteins, such as H-type lactate dehydrogenase (heart specific enzyme) can be
detected in blood.
29