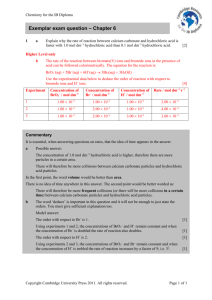
Answers Exam 4 Chem 206 Section B Williamsen MnO4 (aq) + Br
advertisement

Answers
Exam 4
Chem 206 Section B
Williamsen
Potentially Useful Information:
R = 8.31451
J
L ⋅ atm
= 0.0820578
mol ⋅ K
mol ⋅ K
F = 96,485.3
C
mol e −
1 J = 1C × 1 V
1) Balance the following redox reaction. You can use either method.
MnO4–(aq) + Br–(aq)
MnO2(s) + BrO3–(aq)
a) <10 pts.> In acidic solution.
You can use one method or the other.
First the oxidation number method.
{First step: Balance all non-O or H atoms.}
MnO4–(aq) + Br–(aq)
MnO2(s) + BrO3–(aq)
{Second step: Assign oxidation numbers}
Reactants: Mn +7 and Br –1
Products: Mn +4 and Br +5
Multiply Mn-containing species by 2 to balance change in oxidation number.
2MnO4–(aq) + Br–(aq)
2MnO2(s) + BrO3–(aq)
{Balance O's by adding H2O and H's by adding H+}
2MnO2(s) + BrO3–(aq) + H2O(l)
2H+(aq) + 2MnO4–(aq) + Br–(aq)
Second the half-reaction method.
{First step: Write the half-reactions and balance all non-H or O atoms.}
–
Reduction: MnO4 (aq)
MnO2(s)
BrO3–(aq)
–
Oxidation: Br (aq)
{Second step: Balance both half-reactions in H or O atoms.}
+
–
Reduction: 4H (aq) + MnO4 (aq)
3H O(l) + Br–(aq)
2
Oxidation:
{Third step: Balance e–.}
–
MnO2(s) + 2H2O(l)
BrO3–(aq) + 6H+(aq)
–
+
MnO2(s) + 2H2O(l))2
Reduction: (3e + 4H (aq) + MnO4 (aq)
–
3H O(l) + Br (aq)
2
Oxidation:
{Add the two half-reactions together.}
BrO3–(aq) + 6H+(aq) + 6e–
2MnO2(s) + BrO3–(aq) + H2O(l)
2H+(aq) + 2MnO4–(aq) + Br–(aq)
b) <4 pts.> In basic solution.
With whichever method that you use, add OH– to both sides to neutralize H+, make H2O, and cancel H2O that
is on both sides.
2OH–(aq) + 2H+(aq) + 2MnO4–(aq) + Br–(aq)
–
–
Final Answer: H2O(l) + 2MnO4 (aq) + Br (aq)
1
2MnO2(s) + BrO3–(aq) + H2O(l) + 2OH–(aq)
2MnO2(s) + BrO3–(aq) + 2OH–(aq)
2) You have one beaker that contains Cd2+(aq) and a Cd(s) electrode and another beaker with Fe2+(aq) and a Fe(s)
electrode. You connect the two beakers with a salt bridge.
a) <8 pts.> If all components are under standard conditions and you form an electrolytic cell, which half-reaction is
the reduction half-reaction and which half-reaction is the oxidation half-reaction? Why?
The Cd2+/Cd reduction potential is –0.40 V, while the reduction potential for Fe2+/Fe is –0.45 V. Therefore, Fe2+
will be reduced to Fe, and Cd will be oxidized to Cd2+. For an electrolytic cell the standard cell potential must be
negative. This will occur if the reduction occurs from the reaction with the most negative standard reduction
potential and if the oxidation occurs from the reaction with the less negative standard reduction potential.
b) <12 pts.> Draw a picture of the electrolytic cell described in section a). In your picture show all the important
components, label the cathode and anode, label the charge on each electrode, and show in which direction the
cations go through the salt bridge and in which direction the anions go through the salt bridge.
V
Cations
Anions
Cd/Cd2+
Fe2+/Fe
Anode: +
Cathode: –
V is a voltage source.
c) <4 pts.> Write the cell notation for this electrolytic cell.
Cd(s)|Cd2+(aq)||Fe2+(aq)|Fe(s)
d) <7 pts.> What is the standard free energy for this cell? Does the sign of the free energy make sense? Why?
∆G o = − nFE o
96485.3 C
1J
1 kJ
∆G o = −2 mol e − ×
× −0.05 V ×
×
= 9.6 kJ
−
C ⋅ V 1000 J
mol e
The change in standard free energy should be positive for a nonspontaneous reaction. The calculated value makes
sense.
e) <8.5 pts.> Instead of having all species under standard conditions, the Cd2+ solution is 0.30 M and Fe2+ solution is
0.010 M, and the temperature of the cell is 40.0 ºC. Is the cell still electrolytic or is galvanic? Explain your
answer.
Must solve the Nernst equation.
E = Eo −
8.3145 J mol ⋅ K × (273.15 + 40.0 )K
0.30 M
RT
ln Q = −0.05 V −
ln
= −0.10 V
−
−
nF
2 mol e × 96485.3 C mol e × (1 J C ⋅ V ) 0.010 M
The cell is still electrolytic as the cell potential is negative.
2
3) <4 pts.> For each of the following situations, provide the correct sign for the change of enthalpy or change in entropy.
For the problems, just provide the answer.
a) Sign of entropy: A(g) + 2 B(g) à C(g) + 2 D(l) where A, B, C, and D are molecules
Negative. {Products have less entropy. Although there are the same number of molecules on each side, you are
going from three molecules of gas to one of gas and two of liquid.}
b) Sign of entropy: O2 at 25 ºC and 2 atm or O2 at 40 ºC and 2 atm
<Note: Any answer will receive credit as the question is bad> I intended to ask which had more entropy. It
would be the one at higher temperature, because the other conditions are the same in each case.
4) You are studying the following reaction.
4KClO 3 (s) → 3KClO 4 (s) + KCl(s)
a) <6 pts.> Calculate ∆H
o
rxn
for this reaction.
∆H orxn = ((3 mol × −432.75 kJ mol) + (1 mol × −436.7 kJ mol) − (4 mol × −397.7 kJ mol) = −144.2 kJ
b) <6 pts.> Under standard conditions at 25 °C, ∆Sototal =446.4 J/K. Determine ∆Sorxn and ∆Sosurroundings from only the
information given in parts a) and b). Show your work.
∆Sosurroundings = −
− 144.2 kJ 1000 J
∆H
=−
×
= 483.48 J K
T
298.15 K
1 kJ
∆Sorxn = ∆Sototal − ∆Sosurroundings = 446.4 J K − 483.48 J K = −37.1 J K
c) <7 pts.> Is this reaction spontaneous as written at 25 °C? Why?
∆G orxn = ∆H orxn − T∆S orxn = −144.2 kJ − (298.15 K × −37.1 J K ×
1 kJ
) = −133 kJ
1000 J
You can also calculate using ∆G of .
∆G of = (3 mol × −303.2 kJ mol + 1 mol × −409.2 kJ mol) − (4 mol × −296.3 kJ mol) = −133.6 kJ
Reaction is spontaneous because the change in standard free energy is negative.
d) <3.5pts.> What is the equilibrium constant for this reaction at 25.0 °C?
∆G = − RT ln K or K = e
o
−
K=e
−133 kJ
æ
1 kJ ö
çç 8.31451J K × 298.15 K ×
÷
1000 J ÷ø
è
−
∆G o
RT
= 2.0 × 10 23
e) <7 pts.> Is this reaction spontaneous as written at all temperatures? If not, at what temperature does the reaction
become nonspontaneous?
The reaction is not spontaneous at all temperatures as the signs for the changes in entropy and enthalpy are the
same.
T=
∆H o
=
∆So
− 144.2 kJ
= 3887 K
1 kJ
− 37.1 J K ×
1000 J
5) <10 pts.> What are the signs for (+, –, 0) of ∆H and ∆S for the condensation of a vapor (gas) to a liquid? Explain.
∆H: negative and ∆S: negative. The change in enthalpy is exothermic because more attractive interactions are formed
in going from gas to a liquid. The change in entropy is also negative as increased order occurs in going from the
disordered vapor state to a more ordered liquid state.
3
6) <3 pts.> Briefly define what enthalpy is.
Heat produced or taken in under constant pressure conditions
4