Ants in Your Pants? Exploring Alternative Treatment Options for
advertisement

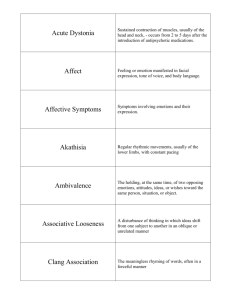
November Ants in Your Pants? Exploring Alternative Treatment Options for Acute Antipsychotic-­‐Induced Akathisia Brittany Finocchio, Pharm.D. PGY-­‐2 Psychiatric Pharmacy Resident The University of Texas at Austin College of Pharmacy Learning Objectives Discuss proposed mechanisms for antipsychotic-­‐induced akathisia (AIA) List risk factors for the development of akathisia secondary to antipsychotic pharmacotherapy Identify traditional agents utilized in the management of acute antipsychotic-­‐induced akathisia Describe the rationale for the use of 5-­‐HT1D agonists and 5-­‐HT2A antagonists in the treatment of acute AIA N o v e m b e r 2 1 , 2 0 1 4 21 WHAT IS AKATHISIA?1-­‐3 I. Extrapyramidal Symptoms a. Dystonia/dyskinesia i. Dystonic reaction 1. Sustained posture from continuous muscle contraction 2. Most common in tongue, jaw, throat, neck, eyes, back, and extremities ii. Dyskinesia 1. Repetitive, abnormal, involuntary jerking movements 2. Typically involves lower face and distal extremities b. Pseudoparkinsonism i. Antipsychotic-­‐induced parkinsonian symptoms 1. Bradykinesia (slowed movement) 2. Tremor (typically while at rest) 3. Cogwheel rigidity c. Akathisia i. Subjective feeling of inner restlessness ii. Objective inability to sit/stand still SYMPTOMS OF AKATHISIA4,5 I. Subjective symptoms a. Impatience b. Irritability c. Panic d. Tension Objective symptoms a. Rocking b. Crossing/uncrossing legs c. Fidgeting of legs d. Myoclonic jerks of feet e. Pacing f. Shuffling of feet g. Shifting weight II. AKATHISIA SUBTYPES5,6 Table 1. Descriptions of Akathisia Subtypes Subtype Description Pseudoakathisia Presence of objective symptoms without subjective symptoms Onset within hours to weeks after treatment initiation or dosage Acute Akathisia increase Chronic Akathisia Variable onset with symptoms that persist for more than 3-­‐6 months Delayed onset (usually 6 weeks to 3 months), with no change in Tardive Akathisia causative medication/dose within 6 weeks of onset Withdrawal Akathisia Onset within 6 weeks of drug discontinuation or dosage decrease 1 Finocchio PATHOPHYSIOLOGY OF AIA3,7-­‐11 I. Imbalance between dopaminergic, and noradrenergic and serotonergic systems a. Dopamine (DA) blockade via norepinephrine (NE) hyperactivity b. Inhibition of DA release via serotonin (5-­‐HT)-­‐2 activity c. Direct blockade of D2 dopaminergic receptors Other associated neurochemicals a. Acetylcholine (ACh) b. Gamma-­‐aminobutyric acid (GABA) c. Neuropeptides II. Figure 1. Select Brain Regions Involved in Dopaminergic Neurotransmission12 a=nigrostriatal pathway, b=mesolimbic pathway, c=mesocortical pathway, d=tuberoinfundibular pathway, e=multi-­‐site dopamine pathway, DLPFC=dorsolateral prefrontal cortex, VMFC=ventromedial prefrontal cortex AGENT-­‐SPECIFIC RISK OF AIA4,8,13-­‐19 I. II. First generation antipsychotics (FGAs): 25% Second generation antipsychotics (SGAs) a. Lurasidone (Latuda): 15% b. Aripiprazole (Abilify): 9-­‐15% c. Risperidone (Risperdal): 13% d. Ziprasidone (Geodon): 7-­‐24% e. Paliperidone (Invega): 7% f. Asenapine (Saphris): 6% 2 Finocchio g. h. i. j. Quetiapine (Seroquel): 3-­‐10% Olanzapine (Zyprexa): 3-­‐7% Clozapine (Clozaril): 3% Iloperidone (Fanapt): 1% RISK FACTORS FOR AIA6,8,9,20,21 Table 2. Risk Factors Associated with the Development of AIA Antipsychotic-­‐Related Risk Factors Other Risk Factors High dose High potency Rapid titration Increased duration of use Parenteral administration Previous akathisia Presence of other EPS Use of other psychotropics Use of other causative agents Affective disorders Negative symptoms Cognitive dysfunction Substance abuse Iron deficiency Development of objeceve motor symptoms Akathisic distress Antipsychotic d ose Subjeceve akathisia Figure 2. Dose-­‐Associated Symptomatology of AIA DIFFERENTIAL DIAGNOSES2,4,9,22,23 I. II. III. IV. V. VI. VII. Agitation Activation Anxiety Neuropsychiatric disorders Neuroleptic rebound syndrome Restless leg syndrome Periodic limb movement disorder 3 Finocchio COMPLICATIONS OF AKATHISIA2,20,24,25 I. II. III. IV. V. VI. VII. Insomnia Suicidality Maladaptive behaviors Tardive dyskinesia Worsening psychosis Non-­‐compliance Impaired treatment response BARNES AKATHISIA RATING SCALE (BARS)4,26,27 I. Most commonly used scale for akathisia diagnosis a. Established validity and high inter-­‐rater reliability Scale components a. Subjective and objective subscales i. May be used to detect change in symptoms with treatment b. Global subscale i. Most appropriate indicator of severity in clinical/research settings ii. May be used to estimate incidence/prevalence II. MANAGEMENT OF AIA8,23 I. Prevention of AIA a. Initiation of lower risk antipsychotics b. Slow dose titration to lowest therapeutic dose Treatment of AIA a. Modify antipsychotic pharmacotherapy a. Initiate anti-­‐akathisia agent II. RECEPTOR PHARMACOLOGY OF ANTI-­‐AKATHISIA THERAPY28-­‐33 Table 3. Receptor Pharmacology of Supported Anti-­‐Akathisia Therapyγ Agent Receptor Site * β * α1,2 Propranolol Benzodiazepines Anticholinergics Cyproheptadine Mirtazapine Trazodone Zolmitriptan X γ X X H1 BZD M D2 X X X X X X X X X X 5-­‐ HT1A 5-­‐ HT1B 5-­‐ HT1D 5-­‐ HT2A 5-­‐ HT2C 5-­‐ HT3 X X X X X X X X X X X X X X X This is not an all-­‐inclusive list, *adrenergic receptors H=histamine, BZD= benzodiazepine, M= muscarinic, D=dopaminergic, 5-­‐HT=serotonergic, SERT=serotonin transporter, DAT=dopamine transporter, NET=norepinephrine transporter 4 Finocchio SERT DAT NET X TRADITIONAL TREATMENT OPTIONS FOR AIA3-­‐5,8,22-­‐24,34-­‐37 I. Propranolol (Inderal) a. Dosing i. Initial: 10 mg by mouth three times daily ii. Max: 120 mg/day b. Evidence for use i. Efficacy rates of 30-­‐70% reported in practice ii. 2004 Cochrane Review 1. Insufficient data for management of akathisia c. Adverse effects i. Orthostasis ii. Bradycardia d. Contraindications/precautions i. Asthma ii. Cardiac conduction abnormalities Benzodiazepines a. Agents i. Clonazepam (Klonopin) 1. Dosing: 0.5-­‐3 mg/day ii. Diazepam (Valium) 1. Dosing: 5-­‐15 mg/day iii. Lorazepam (Ativan) 1. Dosing: 1-­‐2 mg/day b. Evidence for use i. 1999 Cochrane Review 1. May reduce AIA symptoms over a short follow-­‐up period ii. Greater efficacy with clonazepam and lorazepam vs. diazepam c. Adverse effects i. Central nervous system effects ii. Respiratory depression iii. Risk of tolerance/dependence d. Contraindications/precautions i. Use cautiously in patients with hepatic insufficiency ii. Avoid in geriatric patients when possible Anticholinergics a. Agents i. Benztropine (Cogentin) 1. Dosing a. 2-­‐8 mg/day b. Given daily or in 2-­‐3 divided doses ii. Trihexyphenidyl (Artane) 1. Dosing a. 2-­‐10 mg/day b. Divided into 3-­‐4 doses/day b. Evidence for use i. 2002 Cochrane Review 1. Evidence for use of anticholinergics in AIA is controversial II. III. 5 Finocchio ii. 39-­‐73% response rate in studies iii. Most efficacy seen in patients with concomitant parkinsonism and patients on concomitant benzodiazepine therapy c. Adverse effects i. Blurred vision ii. Constipation iii. Xerostomia iv. Cognitive impairment v. Sedation vi. Tachycardia vii. Urinary retention d. Contraindications/precautions i. Use cautiously in patients with tardive dyskinesia, hepatic impairment, renal impairment, benign prostatic hypertrophy, cardiovascular disease, and gastrointestinal obstruction ii. Avoid in patients with narrow-­‐angle glaucoma NEWER ALTERNATIVES FOR AIA8,14,24,35,36,38,39,41 I. 5-­‐HT2A Antagonists a. Agents i. Cyproheptadine (Periactin) 1. Dosing: 4 mg by mouth four times daily 2. Evidence for use: comparable efficacy to propranolol 3. Adverse effects a. Sedation b. Weight gain c. Gastrointestinal adverse effects 4. Contraindications/precautions a. Contraindicated in patients who are breastfeeding, those who are on a monoamine oxidase inhibitor (MAOI), and in patients with narrow-­‐angle glaucoma, urinary obstruction, and stenosing peptic ulcers ii. Mirtazapine (Remeron) 1. Adverse effects a. Sedation b. Weight gain c. Constipation 2. Contraindications/precautions a. Risk of affective switch when used in patients with bipolar disorder b. High doses associated with akathisia development c. Rapid titration and initiation at high doses associated with increased suicidality in youth d. Avoid concomitant use with MAOIs iii. Trazodone (Desyrel) 1. Adverse effects a. Sedation 6 Finocchio b. Orthostasis c. Nightmares d. Priapism 2. Contraindications/precautions a. Risk of affective switch when used in patients with bipolar disorder b. Rapid titration and initiation at high doses associated with increased suicidality in youth c. Avoid concomitant use with MAOIs II. 5-­‐HT1D agonists a. Zolmitriptan (Zomig) b. Adverse effects i. Nausea ii. Dizziness iii. Paresthesia/hyperesthesia/asthenia iv. Sedation v. Cardiac adverse effects c. Contraindications/precautions i. Contraindicated in patients with significant underlying cardiovascular disease, peripheral vascular disease, history of stroke or hemiplegic or basilar migraine, and concomitant MAOI Vitamin B6 (Pyridoxine) a. Dosing i. 600 mg by mouth twice daily b. Mechanism of action i. Co-­‐factor for decarboxylation of dopa to dopamine ii. Required for function of enzymes involved in serotonin, GABA, and melatonin synthesis iii. Possesses antioxidant properties c. Evidence for use i. Shown to be effective when compared to placebo for improvement of BARS subjective and global subscales, with a trend toward improvement in the BARS objective subscale d. Adverse effects i. Headache ii. Nausea e. Contraindications/precautions i. Caution should be exercised in patients with renal dysfunction, as certain intravenous formulations of pyridoxine contains aluminum ii. Large intravenous doses are associated with an increased risk of seizure III. AGENTS WITH LIMITED EFFICACY IN THE MANAGEMENT OF AIA4,5,23,33,42 I. II. III. IV. V. Opiates Clonidine (Catapres) Amantadine (Symmetrel) Ropinirole (Requip) Buspirone (Buspar) 7 Finocchio VI. VII. VIII. IX. X. Fluvoxamine (Luvox) Gabapentin (Neurontin) Iron supplementation Nicotine patches (Nicoderm CQ) Tricyclic antidepressants AGENTS LACKING EFFICACY IN THE MANAGEMENT OF AIA23,43 I. II. Granisetron (Granisol, Sancuso) Valproic acid (Depakene) COURSE OF THERAPY44 I. II. III. Response typically seen within a few days Anti-­‐akathisia therapy slowly withdrawn after weeks to months of treatment Long-­‐term therapy considered if symptoms recur after treatment discontinued LITERATURE REVIEW Poyurovsky M, et al.24 I. Low Dose Mirtazapine: A New Option in the Treatment of Antipsychotic-­‐Induced Akathisia. A Randomized, Double-­‐Blind, Placebo-­‐ and Propranolol-­‐Controlled Trial To determine mirtazapine’s efficacy and tolerability in the treatment of akathisia Study secondary to typical antipsychotics, when compared to the current first-­‐line Objective treatment option for akathisia, propranolol • Psychiatric inpatients Inclusion Criteria • Score of ≥ 2 on the BARS • Diagnosis of acute AIA supported by DSM-­‐IV criteria • Contraindications to beta-­‐blockers • Treatment of emergent akathisia prior to screening Exclusion Criteria • Diagnosis of non-­‐acute akathisia • Treatment with long-­‐acting antipsychotics • Change in AP regimen within 3 days of akathisia onset • Mirtazapine 15 mg by mouth once daily (n=30) Treatment Arms • Propranolol 40 mg by mouth twice daily (n=30) • Placebo (n=30) • Randomization via table of random numbers • Allocation using randomized block design • Identical capsules dispensed by study pharmacist twice daily • BARS conducted at baseline, day 3, and day 7 Methodology • Simpson-­‐Angus Scale (SAS), Brief Psychiatric Rating Scale (BPRS), and Hamilton Rating Scale for Depression (HAM-­‐D) conducted at baseline and on day 7 • Adverse effects assessed daily 8 Finocchio Primary Outcomes Secondary Outcomes • • • • • • Statistical Analysis • • Study Population • Between-­‐group differences in BARS global scores Between-­‐group differences in proportion of responders Between-­‐group difference in psychometric scale score change Between-­‐group difference in adverse events Intention to treat analysis 30 patients per group to achieve 90% power to detect a 40% difference in response rate between treatment and placebo at a significance level of 0.05, with a 25% attrition rate Analysis of between-­‐group differences in demographic and clinical variables, and in changes in psychometric scale scores with ANOVA or Chi-­‐ Square, as appropriate No statistically significant difference in baseline characteristics between groups (mostly men with schizophrenic disorders treated with haloperidol, average age=~34 years old) Over 20 dropouts Table 4. Mean Change in BARS Global Subscale Mirtazapine Propranolol Placebo Mirtazapine Propranolol (n=30) (n=30) (n=30) vs. Placebo vs. Placebo (95% CI, p) (95% CI, p) Baseline -­‐0.77 -­‐0.57 -­‐0.67 -­‐.1 (-­‐0.63 to -­‐.1(-­‐0.35 to to Day 3 0.43, 0.71) 0.55, 0.66) Day 3 to 7 -­‐0.33 -­‐0.23 0.3 -­‐0.63 (-­‐1.05 -­‐0.53 (-­‐0.94 to -­‐0.22, to -­‐0.13, 0.0032) 0.0108) Responder 13 (43.3) 9 (30) 2 (6.7) 10.7 (2.2 to 6 (1.1 to Rate (%) 53.4, 30.7, 0.0010) 0.0195) NNT 3 (1.8 to 4 (2.4 to 5.9) 21.4) Efficacy BARS=Barnes Akathisia Rating Scale, NNT=number needed to treat • • • • Safety Strengths 9 Finocchio • • • • • • Overall reductions in the BARS subscales from baseline to day 7 were 34% in the mirtazapine group, 29% in the propranolol group, and 11% in the placebo group No significant difference in BARS subscales between groups No between-­‐group difference for change in SAS, BPRS, HAM-­‐D Higher incidence of drowsiness in the mirtazapine group (36.7%) vs. 26.6% with propranolol and 20% with placebo 16.7% of patients in the propranolol group prematurely discontinued the study due to hypotension/bradycardia Comparison against placebo and active comparator Use of intention-­‐to-­‐treat analysis One trained research psychiatrist performed all rating scales Scales performed prior to medication administration Magnitude of change in BARS global scales for mirtazapine and Limitations Conclusions propranolol were similar to those seen in previous studies • Short trial duration (7 days) and small sample size • Failure to mention when current AP regimens initiated • Discrepancy between number of dropouts in figure vs. text • Focus on akathisia induced by typical antipsychotics • Continuation of anticholinergics/BZDs initiated prior to study Mirtazapine is a promising alternative to propranolol for the treatment of AIA, considering its comparable efficacy, greater tolerability, and more convenient dosing Stryjer R, et al.39 Trazodone for the Treatment of Neuroleptic-­‐Induced Acute Akathisia: A Placebo-­‐Controlled, Double-­‐Blind, Crossover Study To further evaluate the efficacy of trazodone for the treatment of AIA in a Study objective controlled clinical trial • Inpatients between 18 and 60 years old • Diagnosis of schizophrenia or schizoaffective disorder confirmed by DSM-­‐IV criteria Inclusion Criteria • Treatment with same antipsychotic for ≥ 3 months • Presence of akathisia, confirmed by a BARS score of ≥ 2 • Treatment with beta-­‐blockers, anticholinergics, or BZDs Exclusion Criteria • Presence of any unstable medical condition • Trazodone 100 mg by mouth each night at bedtime on days 1-­‐3, then placebo by mouth at bedtime on days 4-­‐6 (n=8) Treatment Arms • Placebo by mouth each night at bedtime on days 1-­‐3, then trazodone 100 mg by mouth at bedtime on days 4-­‐6 (n=5) • Randomization to trazodone 100 mg days 1-­‐3, then placebo days 4-­‐ 6, or placebo days 1-­‐3, then trazodone 100 mg days 4-­‐6 • No washout period between treatments Methodology • Trazodone and placebo administered in identical capsules • Assessment via the BARS global subscale, SAS, HAM-­‐D, and Positive and Negative Syndrome Scale (PANSS) for schizophrenia by a single rater at baseline and on days 3 and 6 • Treatment effect Study Outcomes • Period effect • Between-­‐group differences in adverse effects • Baseline characteristics between groups compared using paired t tests • Two-­‐tailed paired t tests to assess treatment and period effects Statistical • Effect size calculated via Cohen’s method Analysis • Treatment by group interactions analyzed via 2X2 repeated measures analysis of variance models with between-­‐subjects factor of treatment and within-­‐subject factor of days II. 10 Finocchio Study Population • • • • • Included 7 men and 6 women Mean age = 43 years old 9 patients with schizophrenia 4 patients with schizoaffective disorder No significant differences in outcome measures at baseline Efficacy Safety Strengths Limitations Conclusions Effect Size BARS 1.21 Objective BARS -­‐0.15 ± 0.9 -­‐1.31 ± 0.95 2.74* 1.25 Subjective Awareness BARS 0.08 ± 0.76 -­‐1 ± 0.82 3.74¥ 1.16 Subjective Distress BARS Global 0 ± 1.08 -­‐1.69 ± 1.18 3.39¥ 1.49 SAS -­‐1.84 ± 3.96 -­‐1.54 ± 4.89 0.14 0.07 HAM-­‐D -­‐0.23 ± 2.74 -­‐1.61 ± 2.75 0.99 0.5 PANSS -­‐3.69 ± 7.65 -­‐3.15 ± 8.54 0.14 0.07 * p< 0.05, 2 tailed paired t tests, ¥p < 0.01, 2 tailed paired t tests BARS=Barnes Akathisia Rating Scale, SAS=Simpson Angus Scale, HAM-­‐ D=Hamilton Rating Scale for depression, PANSS= Positive and Negative Syndrome Scale • Significant treatment by day interactions (days 1-­‐3) of the subjective (f1,11=5.39, p=0.04), distress (f1,11=5.31, p=0.04), and global (f1,11=8.13, p=0.016) scores • Significant treatment by day interactions (days 3-­‐6) of distress (f1,11=6.14, p=0.03), and objective (f1,11=5.31, p=0.04) scores • No clinically significant adverse effects in either group • Placebo-­‐controlled • Scales performed by a single rater • Antipsychotic doses remained stable during the study period • Validated previously conducted open-­‐label study • Short trial duration (6 days) • Small sample size • Failure to mention when current AP regimens initiated • Lack of a washout period • Method of randomization not addressed • Method of assessment for adverse effects not discussed • Information on antipsychotic regimens not provided Trazodone is an effective treatment for AIA compared to placebo 11 Finocchio Table 5. Effects of Trazodone vs. Placebo Placebo Trazodone T12 (Mean ± (Mean ± Standard Standard Deviation) Deviation) -­‐0.15 ± 0.69 -­‐1 ± 0.71 2.86* Avital A, et al.36 III. Zolmitriptan compared to propranolol in the treatment of acute neuroleptic-­‐induced akathisia: A comparative double-­‐blind study To further evaluate the efficacy of zolmitriptan for the treatment of AIA in a Study Objective controlled clinical trial, and in comparison to the traditional first-­‐line treatment option for AIA, propranolol. • Inpatient admission for psychotic exacerbation of schizophrenia or schizoaffective disorder Inclusion Criteria • Diagnosis of AIA confirmed by DSM-­‐IV-­‐TR • Minimal BARS score of 5 • Stable antipsychotic dose for ≥ 3 days prior to study initiation • Age < 18 years old or > 60 years old Exclusion Criteria • Significant, concomitant cardiovascular disease • Zolmitriptan 2.5 mg by mouth three times daily (n=14) Treatment Arms • Propranolol 40 mg by mouth three times daily (n=19) • Randomization via a table of random numbers • All patients received identical capsules for 3 consecutive days • Antipsychotic doses remained unchanged throughout study Methodology • BARS, SAS, HAM-­‐D, HAM-­‐A, and PANSS conducted 1 day prior to study initiation (day 0), day 3, and day 7 • Pulse and blood pressure measured twice daily during study • Change in BARS, HAM-­‐D, Hamilton Rating Scale for Anxiety (HAM-­‐ Study Outcomes A), SAS, and PANSS across groups • Between-­‐group differences in pulse (HR)/blood pressure (BP) • Fisher’s Exact Test, General Linear Model (GLM), and unpaired and Statistical paired student’s t-­‐test with time as the within factor and group as Analysis the between factor • Contrasts analysis • Study completed by 8 patients on zolmitriptan and 14 patients on propranolol • No statistically significant differences in baseline characteristics or Study Population outcome measures between groups or between completers and non-­‐completers at baseline • In both groups, most patients were males and had schizophrenia, and average age was between 35-­‐38 years old • Mean BARS scores decreased significantly across both groups between days 0 and 3 (p<0.002) and increased significantly across both groups from days 3 to 7 (p<0.028) • A 4.07 point (39.5%) decrease was observed in the propranolol group from days 0 to 3, compared to a 3.85 point (26.1%) decrease Efficacy in the zolmitriptan group • GLM analysis showed a significant effect for time (F2,36=9.199, P < 0.001); however, effects for group and the interaction between group and time were not significant • Significant reductions in SAS, HAM-­‐D, HAM-­‐A, and PANSS in each 12 Finocchio Safety Strengths Limitations Conclusions group, with no interaction between group and time, and no significant change between groups • No significant difference in HR and BP between groups • One propranolol patient dropped out of study secondary to bradycardia (HR of 45 beats/minute) • Use of an active comparator • Inclusion of patients on a large variety of antipsychotics • Validated previously conducted open-­‐label study • Short trial duration (7 days) • Failure to mention when current AP regimens initiated • Small sample size and no mention of power analysis • Continuation of BZDs and anticholinergics during study • Inclusion of patients on depot injections • Lack of a placebo arm • Study design does not match principles of clinical practice • Incorrect scoring technique for BARS • Use of more than one rater to determine psychometric scale scores Zolmitriptan is equally as effective as propranolol in treating AIA SELECTION OF AN ANTI-­‐AKATHISIA AGENT General Management of Acute AIA Beta-­‐Blocker propranolol 40-­‐120 mg/day Continued Distress 5-­‐HT2A Antagonist mirtazapine 15 mg/day trazodone 100 mg/day cyproheptadine 8-­‐16 mg/day Treatment Failure Addihon of or Change to Benzodiazepine lorazepam 1-­‐2 mg/day clonazepam 0.5-­‐3 mg/day diazepam 5-­‐15 mg/day Use of Alternahve Agent pyridoxine 1200 mg/day zolmitriptan 7.5 mg/day limited efficacy agents Concomitant Parkinsonism Anhcholinergic Monotherapy benztropine 2-­‐8 mg/day trihexyphenidyl 2-­‐10 mg/ day Figure 3. General Management of Acute AIA 13 Finocchio Selechng a First-­‐Line Agent for Acute AIA in Special Populahons* Pseudo-­‐ Parkinsonism Risk of Affechve Switch Desire to Avoid Metabolic Effects Bradycardia or Severe Asthma Current MAOI Use propranolol or anecholinergic propranolol or cyproheptadine propranolol or trazodone 5-­‐HT2A antagonist propranolol MAOI=monoamine oxidase inhibitor *If a patient is unable to use one of the recommended first-­‐line agents, consideration should be given to initiation of a second-­‐line alternative Figure 4. Selecting a First-­‐Line Agent for Acute AIA in Special Populations SUMMARY I. II. III. IV. AIA is difficult to recognize and distressing to the patient Traditional treatment options for AIA have limited data supporting their efficacy Newer treatment options aid clinicians in understanding AIA pathophysiology As the mechanism of AIA further unravels, more efficacious treatment options may be discovered REFERENCES 1. Pierre JM. Extrapyramidal Symptoms with Atypical Antipsychotics: Incidence, Prevention and Management. Drug Safety. 2005;28(3):191-­‐208. 2. Agarkar S, Anthony D, Ferrando S. Risk of Akathisia Associated With Atypical Antipsychotics. J Neuropsychiatry Clin Neurosci. 2013;25(1):E46-­‐E47. 3. Lima AR, Weiser KVS, Bacaltchuk J, Barnes TRE. Anticholinergics for neuroleptic-­‐induced acute akathisia. Cochrane Database of Systematic Reviews 2002, Issue 3. Art. No.: CD003727. DOI: 10.1002/14651858.CD003727.pub2. 4. Nelson DE. Akathisia-­‐A Brief Review. Scot Med J. 2001;46:131-­‐133. 5. Blaisdell GD. Akathisia: A Comprehensive Review and Treatment Summary. Pharmacopsychiat. 1994;27:139-­‐146. 6. Kumar R, Sachdev PS. Akathisia and Second-­‐Generation Antipsychotic Drugs. Curr Opin Psychiatry. 2009;22(3):293-­‐299. 7. Loonen AJM, Stahl SM. The Mechanism of Drug-­‐induced Akathisia. CNS Spectr. 2011;16(1):7-­‐10. 8. Poyurovsky M. Acute antipsychotic-­‐induced akathisia revisited. Br J Psychiatry. 2010;196:89-­‐91. 9. Iqbal N, Lambert T, Masand P. Akathisia: Problem of History or Concern of Today. CNS Spectr. 2007;12(9 Suppl 14):1-­‐16. 10. Furuse T, Hashimoto K. Fluvoxamine for aripiprazole-­‐associated akathisia in patients with schizophrenia: a potential role of sigma-­‐1 receptors. Ann Gen Psychiatry. 2010;9(11):1-­‐3. 14 Finocchio 11. Leong GB, Silva JA. Neuroleptic-­‐Induced Akathisia and Violence: A Review. J Forensic Sci. 2003;48(1):187-­‐189. 12. Stahl SM. Psychosis and Schizophrenia. Stahl’s Essential Psychopharmacology, 3rd ed. Cambridge, NY;Cambridge University Press;2008:247-­‐285. 13. Poyurovsky M, Bergman J, Pashinian A, et al. Beneficial effect of low-­‐dose mirtazapine in acute aripiprazole-­‐induced akathisia. Int Clin Psychopharmacol. 2014;29:296-­‐298. 14. Ribosa-­‐Nogue R, Pagonabarraga J, Kulisevsky J. Efficacy of trazodone in antipsychotic-­‐induced akathisia resistant to conventional treatment. Parkinsonism Rel Disord. 2012;18:902-­‐903. 15. Cutler AJ, Kalali AH, Weiden PJ, et al. Four-­‐Week, Double-­‐Blind, Placebo-­‐and Ziprasidone-­‐ Controlled Trial of Iloperidone in Patients With Acute Exacerbations of Schizophrenia. J Clin Psychopharmacol. 2008;28(2):s20-­‐s28. 16. Rybakowski JK, Vansteelandt K, Remlinger-­‐Molenda A, et al. Extrapyramidal symptoms during treatment of first schizophrenia episode: Results from EUFEST. Eur Neuropsychopharmacol. 2014;24:1500-­‐1505. 17. Citrome L. Asenapine for schizophrenia and bipolar disorder: a review of the efficacy and safety profile for this newly approved sublingually absorbed second-­‐generation antipsychotic. Int J Clin Pract. 2009;63(12):1762-­‐1784. 18. Gopal S, Liu Y, Alphs L, et al. Incidence and time course of extrapyramidal symptoms with long-­‐ acting injectable paliperidone: a posthoc pooled analysis of seven randomized controlled studies. Neuropsychiatr Dis Treat.2013;9:1381-­‐1392. 19. Kurz M, Hummer M, Oberbauer H, et al. Extrapyramidal side effects of clozapine and haloperidol. Psychopharmacology. 1995;118:52-­‐56. 20. Shear MK, Frances A, Weiden P. Suicide Associated with Akathisia and Depot Fluphenazine Treatment. J Clin Psychopharmacol. 1983;3(4):235-­‐236 21. Caroff SN, Hurford I, Lybrand J, et al. Movement Disorders Induced by Antipsychotic Drugs: Implications of the CATIE Schizophrenia Trial. Neurol Clin. 2011;29(1):127-­‐viii. 22. Pfeffer G, Chouinard G, Margolese HC. Gabapentin in the treatment of antipsychotic-­‐induced akathisia in schizophrenia. Int Clin Psychopharmacol. 2005;20:179-­‐181. 23. Miller CH, Fleischhacker WW. Managing Antipsychotic-­‐Induced Acute and Chronic Akathisia. Drug Safety. 2000;22(1):73-­‐81. 24. Poyurovsky M, Pashinian A, Weizman R, et al. Low-­‐Dose Mirtazapine: A New Option in the Treatment of Antipsychotic-­‐Induced Akathisia. A Randomized, Double-­‐Blind, Placebo-­‐ and Propranolol-­‐Controlled Trial. Biol Psychiatry. 2006;59:1071-­‐1077. 25. Berardi D, Giannelli A, Barnes TRE. Clinical correlates of akathisia in acute psychiatric inpatients. Int Clin Psychopharmacol. 2000;15(4):215-­‐219. 26. Barnes TR. A Rating Scale for Drug-­‐Induced Akathisia. Br J Psychiatry. 1989;154:672-­‐676. 27. Barnes TR. The Barnes Akathisia Rating Scale-­‐Revisited. J Psychopharmacol. 2003;17(4):365-­‐370. 28. Stahl SM. Mechanism of Action of Trazodone: a Multifunctional Drug. CNS Spectr. 2009;14(10):536-­‐546. 29. Fischel T, Hermesh H, Aizenberg D, et al. Cyproheptadine Versus Propranolol for the Treatment of Acute Neuroleptic-­‐Induced Akathisia: A Comparative Double-­‐Blind Study. J Clin Psychopharmacol. 2001;21(6):612-­‐615. 30. Boer T. The Pharmacologic Profile of Mirtazapine. J Clin Psychiatry. 1996;57(4):19-­‐25. 31. Anonymous. Lexi-­‐Drugs. (http://online.lexi.com/lco/action/home). Accessed 21 October 2014. 32. Boyer EW. Serotonin syndrome. (http://www.uptodate.com/contents/serotonin-­‐syndrome). Updated 2 July 2014. Accessed 13 November 2014. 33. Sethuram K, Gedzior J. Akathisia: Case Presentation and Review of Newer Treatment Agents. Psychiatr Ann. 2014;44(8):391-­‐396. 15 Finocchio 34. Barnes TRE, Soares-­‐Weiser K, Bacaltchuk J. Central action beta-­‐blockers versus placebo for neuroleptic-­‐induced acute akathisia. Cochrane Database of Systematic Reviews 2004, Issue 4. Art. No.: CD001946. DOI: 10.1002/14651858.CD001946.pub2. 35. Stryjer R, Strous RD, Bar F, et al. Treatment of Neuroleptic-­‐Induced Akathisia with the 5-­‐HT2A Antagonist Trazodone. Clinical Neuropharmacology. 2003;26(3):137-­‐141. 36. Avital A, Gross-­‐Isseroff R, Stryjer R, et al. Zolmitriptan compared to propranolol in the treatment of acute neuroleptic-­‐induced akathisia: A comparative double-­‐blind study. Eur Neuropsychopharm. 2009;19:476-­‐482. 37. Resende Lima A, Soares-­‐Weiser K, Bacaltchuk J, Barnes TRE. Benzodiazepines for neuroleptic-­‐ induced acute akathisia. Cochrane Database of Systematic Reviews 1999, Issue 4. Art. No.: CD001950. DOI: 10.1002/14651858.CD001950. 38. Poyurovsky M, Weizman R, Weizman A. Mirtazapine-­‐A Multifunctional Drug: Low Dose for Akathisia. CNS Spectr. 2011;16(2):63. 39. Stryjer R, Rosenzcwaig S, Bar F, et al. Trazodone for the Treatment of Neuroleptic-­‐Induced Acute Akathisia: A Placebo-­‐Controlled, Double-­‐Blind, Crossover Study. Clinical Neuropharmacology. 2010;33(5):219-­‐222. 40. Lerner V, Bergman J, Statsenko N, et al. Vitamin B6 Treatment in Acute Neuroleptic-­‐Induced Akathisia: A Randomized, Double-­‐Blind, Placebo-­‐Controlled Study. J Clin Psychiatry. 2004;65:1550-­‐1554. 41. Miodownik C, Lerner V, Statsenko N, et al. Vitamin B6 Versus Mianserin and Placebo in Acute Neuroleptic-­‐induced Akathisia: A Randomized, Double-­‐blind, Controlled Study. Clin Neuropharmacol. 2006;29:68-­‐72. 42. Sullivan MA, Wilbur R. Gabapentin pharmacotherapy for antipsychotic-­‐induced akathisia: single-­‐ patient experiment and case report. Ther Adv Psychopharmacol. 2014;4(2):100-­‐102. 43. Poyurovsky M, Weizman A. Lack of efficacy of the 5-­‐HT3 receptor antagonist granisetron in the treatment of acute neuroleptic-­‐induced akathisia. Int Clin Psychopharmacol. 1999;14:357-­‐360. 44. Semple D, Smyth R. Therapeutic Issues. Oxford Handbook of Psychiatry, 3rd Ed. Oxford, United Kingdom;Oxford University Press;2013:923-­‐980. 45. Zacher JL, Holmes JC. Second-­‐generation antipsychotics: A review of recently-­‐approved agents and drugs in the pipeline. Formulary. 2012;47:106–112,119–121. 46. Poyurovsky M, Weizman A. Serotonin-­‐based pharmacotherapy for acute neuroleptic-­‐induced akathisia: a new approach to an old problem. Br J Psychiatry. 2001;179:4-­‐8. 47. Poyurovsky M, Weizman A. Serotonergic Agents in the treatment of acute neuroleptic-­‐induced akathisia: open-­‐label study of buspirone and mianserin. Int Clin Psychopharmacol. 1997;12:263-­‐ 268. 48. Weiss D, Aizenberg D, Hermesh H, et al. Cyproheptadine Treamtent in Neuroleptic-­‐Induced Akathisia. Br J Psychiatry. 1995;167:483-­‐486. 49. Poyurovsky M, Fuchs C, Weizman A. Low-­‐Dose Mianserin in Treatment of Acute Neuroleptic-­‐ Induced Akathisia. J Clin Psychopharmacol. 1998;18(3):253-­‐254. 50. Poyurovsky M, Shardorodsky M, Fuchs C, et al. Treatment of neuroleptic-­‐induced akathisia with the 5-­‐HT2 antagonist mianserin. Double-­‐blind, placebo-­‐controlled study. Br J Psychiatry. 1999;174:238-­‐242. 51. Miller G, Fleischhacker WW, Ehrman H, et al. Treatment of neuroleptic-­‐induced akathisia with the 5-­‐HT2 antagonist ritanserin. Psychopharmacol Bull. 1990;26:373-­‐376. 52. Gross-­‐Isseroff R, Magen A, Shiloh R, et al. The 5-­‐HT1D receptor agonist zolmitriptan for neuroleptic-­‐induced akathisia: an open label preliminary study. Int Clin Psychopharmacol. 2005;20:23-­‐25. 16 Finocchio APPENDIX A: RECEPTOR BINDING AFFINITIES OF ATYPICAL ANTIPSYCHOTICS45 Table 6. Select Receptor Binding Affinities of Second Generation Antipsychotics (SGAs) SGA Receptor Binding Affinity* 5-­‐HT2A D2 H1 M1 α1 Lurasidone 0.47 0.99 10.8 >1000 >1000 Aripiprazole 3.4 0.45 47 1 >1000 Risperidone 0.5 4 0.7 20 >1000 Ziprasidone 0.4 5 11 50 >1000 Paliperidone 0.25 4.6 80 13.6 >1000 Asenapine 0.006 1.3 1.2 1 >1000 Quetiapine 31 770 8.1 19 >1000 Olanzapine 4 11 19 7 1.9 Clozapine 16 126 7 6 1 Iloperidone 5.6 6.3 36 473 >1000 *Receptor binding affinities are expressed as Ki (inhibition constant) values, with the lowest value correlating to the strongest binding affinity. Ki values may vary based on antipsychotic dose. APPENDIX B: BARNES AKATHISIA RATING SCALE (BARS)23 Instructions: Observe patient while seated, and then standing during neutral conversation (minimum of two minutes each). Symptoms observed in other situations may also be rated. Subjective phenomena should be identified via direct questioning. Objective Rating 0 Normal, occasional fidgety movements of the limbs 1 Presence of characteristic restless movements: shuffling or tramping movements of the legs/feet, or swinging of one leg while sitting, and/or rocking from foot to foot or “walking on the spot” when standing, but movements present for less than half the time observed 2 Observed phenomena, as described in (1) above, which are present for at least half the observation period 3 Patient is constantly engaged in characteristic restless movements, and/or has the inability to remain seated or standing without walking or pacing, during the time observed Subjective Rating Awareness of Restlessness 0 Absence of inner restlessness 1 Non-­‐specific sense of inner restlessness 2 The patient is aware of an inability to keep the legs still, or a desire to move the legs, and/or complains of inner restlessness aggravated specifically by being required to stand still 3 Awareness of intense compulsion to move most of the time and/or reports strong desire to walk or pace most of the time 17 Finocchio Subjective Rating Distress Related to Restlessness 0 1 2 3 No distress Mild distress Moderate distress Severe distress 0 1 2 3 4 5 Global Clinical Assessment of Akathisia Absence of akathisia: No evidence of awareness of restlessness. Observation of characteristic movements of akathisia in the absence of a subjective report of inner restlessness or compulsive desire to move the legs should be classified as pseudoakathisia. Questionable akathisia: Non-­‐specific inner tension and fidgety movements. Mild akathisia: Awareness of restlessness in the legs and/or inner restlessness worse when required to stand still. Fidgety movements present, but characteristic restless movements of akathisia not necessarily observed. Condition causes little or no distress. Moderate akathisia: Awareness of restlessness as described for mild akathisia above, combined with characteristic restless movements such as rocking from foot to foot when standing. Patient finds the condition distressing. Marked akathisia: Subjective experience of restlessness includes a compulsive desire to walk or pace. However, the patient is able to remain seated for at least five minutes. The condition is obviously distressing. Severe akathisia: The patient reports a strong compulsion to pace up and down most of the time. Unable to sit or lie down for more than a few minutes. Constant restlessness which is associated with intense distress and insomnia. Scoring the Barnes Akathisia Rating Scale (BARS) Objective and subjective subscales may be summed to provide a total score ranging from 0 to 9. The Global Clinical Assessment of Akathisia is scored separately. APPENDIX C: LITERATURE ON SEROTONERGIC AGENTS FOR TREATMENT OF AIA46 Table 7. Studies of Use of Serotonergic Agents in the Treatment of AIA Total Dose Outcome Medication* Study Design Sample (mg/ Measures Size day) Buspirone47 Open trial Cyproheptadine 48 Cyproheptadine 29 Open trial Double blind vs. propranolol 18 Finocchio 10 17 30 Results 10-­‐30 BARS,SAS 2 improved, 6 unchanged, 2 worsened 16 HAS,BPRS, HAM-­‐D, AIMS 15/17 improved (6/17 complete resolution) 16 BARS,SAS, BRPS 40% BARS decrease w/ cyproheptadine, 42% w/ propranolol Table 7. Studies of Use of Serotonergic Agents in the Treatment of AIA (cont’d) Total Dose Outcome Medication* Study Design Sample (mg/ Resultsγ Measures Size day) BARS,SAS, mLAS, 2 improved, 5 Granisetron43 Open trial 10 2 BRPS, unchanged, 3 dropouts HAM-­‐D Improvement of all 3 Mianserin49 Open trial 16 15 BARS,SAS BARS subscales BARS,SAS 14/15 on mianserin Double blind BPRS, Mianserin50 30 15 improved vs. 5/11 on vs. placebo mLAS, placebo HAM-­‐D 5/8 patients experienced ≥ 2-­‐point decrease on Retrospective BARS global subscale. Mirtazapine13 8 15 BARS chart review One patient had 1 point decrease, another did not respond 34% decrease in BARS Double blind BARS,SAS, global subscale with 24 Mirtazapine vs. 90 15 BPRS, mirtazapine vs. 29% with propranolol HAM-­‐D propranolol and 11% with placebo 3/3 patients with Ritanserin51 Open trial 10 5-­‐20 HAS,CGI resistant AIA improved BARS global scores BARS,SAS, decreased to 0 in 5/9 mLAS, patients. The other 4 Trazodone35 Open trial 9 100 PANSS, HAM-­‐D, patients had decreases ≥ CGI 2 on the global subscale. BARS,SAS, Greater decreases in all Crossover trial Trazodone39 13 100 HAM-­‐D, BARS subscales with vs. placebo PANSS trazodone vs. placebo BARS, BARS significantly Zolmitriptan52 Open trial 8 7.5 PANSS improved in 6/8 patients BARS,SAS, 26.1% decrease in mean Double blind HAM-­‐D, BARS with zolmitriptan Zolmitriptan 36 vs. 33 7.5 HAM-­‐A, vs. 39.5% decrease with propranolol PANSS propranolol γ *This table excludes case reports and may not be an all-­‐inclusive list. Although positive results are reported, careful review of study methodologies is encouraged for adequate interpretation of results. BARS=Barnes Akathisia Rating Scale, SAS=Simpson Angus Scale, HAS=Hillside Akathisia Scale, BPRS=Brief Psychiatric Rating Scale, HAM-­‐D=Hamilton Rating Scale for Depression, AIMS=Abnormal Involuntary Movement Scale, mLAS=modified Leeds Anxiety Scale, CGI=Clinical Global Impression, PANSS=Positive and Negative Syndrome Scale, HAM-­‐A=Hamilton Rating Scale for Anxiety 19 Finocchio