Chemistry 1515L Week 7
advertisement

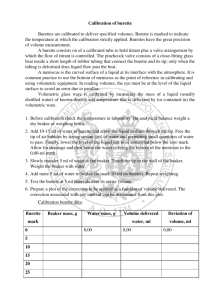
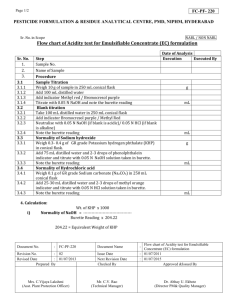
Chemistry 1515L Week 7 Graduate Teaching Assistant Contact Information Standard Solutions: Titration The use of M1V1 = M2V2 to calculate the concentration (molarity) of an unknown acid solution • We make up a solution of NaOH of approximate concentration • We use a burette to add a solution of HCl of exact concentration • Use phenolphthalein as an indicator to find neutralization point p p p • This helps us find the exact concentration of the NaOH solution • We then titrate that NaOH solution with unknown acid We then titrate that NaOH solution with unknown acid • This helps us calculate the concentration of the unknown acid Setting up the Burettes • We We use a burette clamp attached to the ring stand to use a burette clamp attached to the ring stand to support the burettes • Each Each clamp with safely support two burettes, one for clamp with safely support two burettes one for adding acid, the other for adding base Making the NaOH Solution • You You will use the Florence flask to make up the approximate will use the Florence flask to make up the approximate NaOH solution • Swirl the flask gently to make sure that the solution is well mixed Using the Burettes • Pour liquids into burettes over the sink. DO NOT attempt to pour in solutions above your pour in solutions above your eye level as this is very dangerous. • Make sure the flow from the burette is reasonable and that the opening is not blocked. p g • Make sure that the liquid does not splash out of the flask when dropping from the h d i f h burette. More on the Webpage Using the Burettes • Placing a piece of paper behind the burette will help you read the liquid level. the liquid level. • The bottom of the meniscus is the point at which you take the reading. • Make sure you make readings at, or below, eye level. , , y With phenolphthalein indicator, With phenolphthalein indicator adding the solutions will give a bright pink color as the titration progresses You are looking for progresses. You are looking for the color to persist. Calculations The use of M1V1 = M2V2 to calculate the concentration (molarity) of an unknown acid solution A copy of the modified Report Sheet is at the front of the lab Make sure you write in the Unknown Acid number y Waste solutions may be washed down the sink with plenty of water Waste solutions may be washed down the sink with plenty of water For Next Lab Session: • Read all of the material available related to the next experiment (Analysis of a Commercial Antacid). • Email me with any concerns.