SOLUBILITY RULES You should use the following rules to predict
advertisement

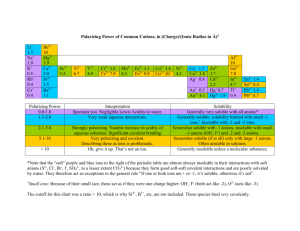
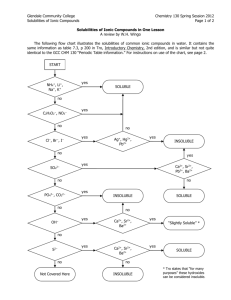
SOLUBILITY RULES You should use the following rules to predict the solubility of ionic compounds in water. These rules are only guidelines, and there are exceptions besides the ones listed. However, the ions normally encountered in an introductory chemistry course, in most cases, will follow these rules. This list of rules is in order of decreasing importance. That is, if the first rule is applicable then follow it regardless of what the other rules may predict. Similarly, if the second rule is applicable then ignore the third rule. Thus, you should only use the third rule when both rules one and two are not applicable. In general, these rules relate to the lattice energies of the particular compounds along with the hydration energies of the individual ions. As a simplification, it is assumed that the greater the lattice energy to lower the solubility. One of the factors determining the lattice energy are the magnitudes of the charges on the ions (the greater the magnitude of the charges the greater the lattice energy). For this reason, the basis of these rules is the magnitudes of the charges. These rules ignore other influences on the lattice energies and the influence of the hydration energy. In addition, the rules disregard the effect of temperature on solubility. 1. Compounds containing an ion with a +1 or -1 ion are normally soluble. Examples: NaBr, K2SO4, FeCl2, AlCl3, Rb3PO4 Exceptions (Insoluble): OH- (other than with cations that produce strong bases) IB metals (column 11) and Hg22+ |(other than with NO3-|C2H3O2-, ClO3-, and |ClO4-) 2+ 2+ | Pb , Hg with most -1 ions 2. Compounds containing an ion with a +3, -3 or higher charge ion are normally not soluble. Examples: AlPO4, TiO2, Ca3(PO4)2 Exceptions (Soluble): Cations combined with sulfate or dichromate. © 2006 Sevagram Enterprises 3. Compounds containing ions with a -2 charge are normally not soluble. Examples: CaCO3, FeS, ZnC2O4 Exceptions (Soluble): Dichromates Sulfates (Insoluble only if combined with Pb, Hg, Ca, Sr, or Ba) Note: The following ions are nearly always soluble: Na+, K+, NH4+, NO3-, C2H3O2-, ClO3- and ClO4Note: Hydrides (H-) decompose in water to yield H2 and OHNote: Many metal oxides will react with water to produce hydroxides. Examples: NaCl FeAsO4 Na3PO4 AgBr Fe2(SO4)3 BaCO3 MgSO4 Ag2S (NH4)2CO3 NaOH AgOH NaClO3 Hg2Cl2 Ba(OH)2 AgCl NH4IO3 PbCl2 Ba(NO3)2 PbCrO4 ZnS BaSO4 AgNO3 Ba(IO3)2 AgIO3 Soluble (Rule 1) Insoluble (Rule 2) Soluble (Rule 1) Insoluble (Rule 1) Soluble (Rule 2) Insoluble (Rule 3) Soluble (Rule 3) Insoluble (Rules 1) Soluble (Rule 1) Soluble (Rule 1) Insoluble (Rule 1) Soluble (Rule 1) Insoluble (Rule 1) Soluble (Rule 1) Insoluble (Rule 1) Soluble (Rule 1) Insoluble (Rule 1) Soluble (Rule 1) Insoluble (Rule 3) Insoluble (Rule 3) Insoluble (Rule 3) Soluble (Rule 1) Insoluble Insoluble (Rule 1) © 2006 Sevagram Enterprises Na+ and ClFe3+ and AsO43Na1+ Ag+ SO42CO32SO42Ag+ NH41+ Strong base Not a strong base Na+ and ClO3Hg22+ Strong base Ag+ NH4+ and IO3Pb2+ NO3CrO42S2Ba2+ + SO42NO3Exception Ag+