presentation - Partnering For Cures
advertisement

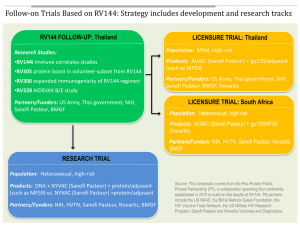
The Michael J. Fox Foundation - Sanofi: Partnering for Parkinson’s Research Todd Sherer, Marc Bonnefoi MJFF’s mission Drive the best Parkinson’s research Deliver improved therapies and a cure MJFF snapshot • MJFF has funded over $304M in research since its founding in 2000 • • • • Over $55M was directed toward PD research in 2011 Estimate ~$55M in grants awarded in 2012 In 2011, we received nearly 64,000 contributions and raised $68.4M Core values are efficiency and accountability: 88 cents of every $1 spent goes straight to research program efforts new commitments in millions 60 50 40 30 20 10 0 2001 • • 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 est Our in-house staff of 7 PhDs, 1 MD and 8 business strategists serve as portfolio managers, incorporating the advice and input of experts from academia and industry into their decision-making We reviewed over 900 PD-specific grants in 2011 and currently have over 450 active grants in our portfolio MJFF’s approach to funding Craft an informed research agenda • Understand patient needs • View global field and identify most promising targets • PhDs balance relevance and merit while business managers balance prioritization and risk Map out critical research plans • Infuse capital at underfunded, high-risk stages • Develop and share essential research tools • Pragmatically push research in a goal directed, milestonedriven fashion Problem-Solve: lead and innovate • Convene experts in non-competitive environment • Facilitate handoffs and orchestrate connections • Showcase top ideas to industry Our history Our commitment Sanofi is more than a pharmaceutical laboratory; we are a diversified healthcare company © Urbanhearts/Fotolia We act with our partners to protect health, enhance life and respond to the potential healthcare needs of the 7 billion people around the world CORPORATE PRESENTATION 2012 Our priority A new R&D model: • Simplification • Openness • Partnerships • Biotechnologies 2012 - 2015 18 potential new launches* Priorities Diabetes Fibrosis and tissue repair Immuno-inflammation Infectious diseases Rare diseases Oncology Ophthalmology Aging R&D Portfolio As of February 2012 60 molecules and vaccines Source: Press Release Feb. 8, 2012 * Scope includes New Molecular entities (excluding life cycle management) and vaccines CORPORATE PRESENTATION 2012 © Imagesource V/Fotolia Accelerate innovations for patients Our core belief: We cannot innovate alone PAYERS PROVIDERS Our partner, MJFF Therapeutics Development Initiative • Nurr1 project Tools/Models • LRRK2 Industry Advisory Group PD trial design • LRRK2 clinical trial design working group Expert network • Advice on non-motor symptoms of PD Drug Positioning • AVE8112 The AVE8112 Story • Sanofi approached MJFF to discuss how to best advance AVE8112 because it had shown promising pro-cognitive activity in preclinical models • MJFF staff, with additional input from external advisors, determined the compound should be tested clinically • Agreement was set up: • MJFF to sponsor a multiple ascending dose phase Ib clinical trial, to assess safety and tolerability of AVE8112 in patients with Parkinson’s disease • Sanofi provides the compound and information for IND application • All data and results owned by MJFF and shared with Sanofi • Further development plans will be based upon the results of the study The AVE8112 Story (cont’d) • Trial details • • • • 32 patients (4 cohorts of 8 patients each) Dose escalation with a planned titration schedule 2 sites (California and Baltimore) Working with Parexel Early Phase Unit (CRO) for trial execution • Progress • MJFF held pre-IND meeting with FDA in August 2012, which Sanofi attended • IND filing in early December 2012 • Start of the clinical trial expected in January 2013 • Final results expected in December 2013 MJFF and AVE8112 • Mission to accelerate treatments for PD • Repositioning compounds → potentially faster delivery of therapy to patients • PD cognition focus for MJFF, based on patients’ needs • De-risk PD for larger groups and funders who have ability to ultimately commercialize