mussel dissection - Marine Biotechnology and Bioinformatics is a
advertisement

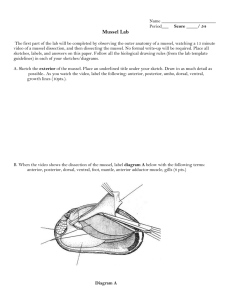
MARINE BIOTECHNOLOGY & BIOINFORMATICS FOR TEACHERS MOSS LANDING MARINE LABS NSF ITEST GRANT MUSSEL DISSECTION Title of Lesson: Dissection of Marine Mussel Designed by: Hilary Hayford and Dr. Simona Bartl (sbartl@mlml.calstate.edu) Safety Scalpel and razor blades should always be used with the blade moving away from you. Blades should be disposed of in an appropriate sharp waste container. Dissection instruments should be used only for there appropriate intended purposes. Materials/Resources • 5% bleach solution in a wash bottle • Paper towels • Weigh boats (1 per mussel) • Razor blades (straight edge, 1 per group) • Dissection kit (1 per group) • Cutting board (1 per group) • Solid waste containers (e.g. plastic 500 ml beakers, 1 per group) NOTE: Mussel tissue can be thrown in the trash. • Sharp waste container (for blades and broken microscope slides) • Gloves (1 pairs per person) • Microscope slide (for tissue examination) • Slide coverslip (for tissue examination) • Compound and dissection microscopes Procedure Pre-lab: 1. Collect mussels. 2. Distribute to each station the following: 1 dissection kit, 1 cutting board, and 1 razor blade per group; 1 weigh boat and 1 microscope slide. Copyright 2006, MLML – NSF ITEST Program, p. 1 Lab: 1. Label each weigh boat using label tape and label the 1.5 mL sample tube. (the tube will be used at the end to collect your mussel DNA) 2. Place each mussel in a clean weigh boat so that it lies on one side, with the narrowest (anterior) part of the shell pointing towards you and the hinge on the left. The hinge is at the umbo (bent) part of the mussel shell. There may be byssal threads protruding from the opposite side. In the box below draw you mussel specimen and LABEL using the following terms: anterior, posterior, dorsal, ventral, and byssal threads Be sure your sketch fills the entire box! 3. Insert the corner of razor blade into the ventral opening opposite the hinge with sharp part of blade pointing away from you. 4. Slice the posterior adductor muscle by moving the blade away from you and towards the wider (posterior) part of the mussel shell. 5. Rock the blade back and forth as you work your way around the posterior end and down to the hinge. Repeat motion moving the blade from opening to hinge until the mussel is severed. You should be able to pull apart the shell valves with your hands once the mussel has been severed. If you have difficulty see an instructor. 6. Once posterior adductor muscle has been severed, open mussel by pulling the two shell valves gently apart. Be gentle, but it is okay if you feel some tissue tearing as you open the mussel. 7. Locate the gills of the mussel. The gills will be on either side of the central organs, lying along the mantle. (The mantle lines each shell valve.) They will be the length of the mussel, peach to beige, with visible striations. Mantle Edge Edge Gonad Cut Adductor Muscle Gill Copyright 2006, MLML – NSF ITEST Program, p. 2 8. Using the forceps, pull off a small amount of gill tissue (about 3-4 rice grains worth) and add to your sample tube. 9. Wash razor blade, scalpel, forceps, and other tools with a 5% bleach solution and dry. 10. For each mussel, find the gill tissue. 11. Make a squash by pinching a small amount (about the size of a pencil tip) of gill tissue onto a microscope slide. Spread the tissue thinly over the slide and place the coverslip over the tissue, pressing firmly. Use a compound microscope to analyze and look at the tissue. Sketch a picture of your slide below 12. Scrape the remaining tissue out of each side of the mussel. Clean and dry the shell. 13. Using a permanent marker, label each shell with your name and date. 14. Measure the length of shell (in centimeters) from the anterior end to the furthest point of the posterior end. Record longest measurement. ___________ cm View of left valve containing all tissues: Dorsal Gill Posterior Gonad Gonopore region Anterior Ventral Copyright 2006, MLML – NSF ITEST Program, p. 3