Biofeedback
Volume 35, Issue 2, pp. 54-61
©Association for Applied Psychophysiology & Biofeedback
www.aapb.org
SPECIAL TOPICS
Is There More to Blood Volume Pulse Than Heart Rate
Variability, Respiratory Sinus Arrhythmia, and
Cardiorespiratory Synchrony?
Erik Peper, PhD,1 Rick Harvey, PhD,1 I-Mei Lin, MA,2 Hana Tylova,1 and Donald Moss, PhD3
1
San Francisco State University, San Francisco, CA; 2National Chung Cheng University, Taiwan; 3Saybrook Graduate School, San Francisco, CA
Keywords: blood volume pulse, heart rate variability, respiratory sinus arrhythmia, cardiorespiratory synchrony
Summer 2007 Ô Biofeedback
A growing body of research reports the health benefits
of training heart rate variability (HRV), and the clinical
use of HRV training protocols has increased dramatically
in recent years. Many of the home training devices and
many of the sophisticated biofeedback instrumentation
systems rely on the blood volume pulse (BVP) sensor, or
photoplethysmograph, because it is more user friendly than
the electrocardiogram used in medical settings. However,
the BVP signal is valuable in its own right, not merely
as a convenient measure of HRV. This article explores the
methodology of BVP recording, the underlying physiology,
and the potential benefits from BVP treatment and training
protocols. For example, the shape of the BVP waveform
reflects arterial changes correlated with hypertension. In
addition, BVP training offers promise for the treatment of
migraine and the monitoring of human sexual arousal.
54
Studies of methods for training improvement in heart
rate variability (HRV), respiratory sinus arrhythmia (RSA),
and cardiorespiratory synchrony (CRS) are promising
areas of current research, expanding our knowledge of new
clinical applications and training procedures for improving
sympathetic-parasympathetic balance (see Table for the
expansion of the abbrevations in text). For example, research
by Lehrer and colleagues (Lehrer et al., 2006; Lehrer et al.,
2004) has demonstrated that HRV training in patients with
asthma reduces their asthma severity. Similarly, research
by Del Pozo and colleagues (Del Pozo, Del Pozo Scher, &
Guarneri, 2004) has shown that cardiac patients can improve
their HRV. In addition, Giardino, Chan, and Borson (2004)
reported a benefit for patients with chronic obstructive
pulmonary disease using HRV training and an exercise
protocol. It appears that learning to increase HRV results
in strengthening sympathetic-parasympathetic balance
and offers hope for many chronic illnesses (Gevirtz, 2003).
Whereas clinical applications and feedback training of RSA
or CRS have been used to address illness affected by the
sympathetic-parasympathetic imbalance, new HRV training
protocols could be employed as well.
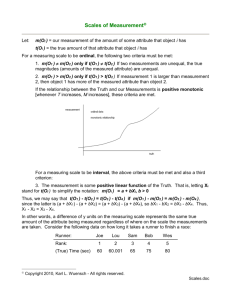
Measuring HRV, the beat-to-beat variability in the sinus
rhythm over a given period of time, is usually done by
calculating the standard deviation of the average of normalto-normal heart beats (SDNN or SDANN). This statistical
index has health implications, predicting morbidity and
mortality. We will elaborate later on this relationship between
HRV and health (Kleiger, Miller, Bigger, & Moss, 1987).
There are many technologies that can estimate the beatto-beat variability in the sinus rhythm over a given period
Table. Alphabet soup of abbreviations
BVA
Blood volume amplitude
BVP
Blood volume pulse
CRS
Cardiorespiratory synchrony
EKG
Electrocardiography
ECG
Electrocardiography
FFT
Fast Fourier transform
HR
Heart rate
HRV
Heart rate variability
NN
Normal-to-normal beat (interbeat interval)
PPG
Photoplethysmography
PTT
Pulse transit time
RSA
Respiratory sinus arrhythmia
SDANN
Standard deviation of average normal-to-normal
beat (also known as SDNN: standard deviation
of the normal-to-normal beat)
SDAHR
Standard deviation of average heart rate
Peper, Harvey, Lin, Tylova, Moss
Figure 1. Blood volume pulse, blood volume amplitude, heart rate, and
respiration before training.
breaths per minute with a corresponding heart rate of 73
beats per minute (SD = 10.1 beats).
Whereas designing training protocols for improving
HRV or RSA/CRS is possible using BVP technologies, the
BVP signal holds even greater promise as a tool for use in
improving other clinical applications and training protocols.
The remainder of this article explores various components
of the BVP technology.
Background
The BVP signal is derived with a PPG sensor that measures
changes in blood volume in arteries and capillaries by shining
an infrared light (a light-emitting diode) through the tissues.
This infrared light is selectively transmitted, backscattered,
reflected, and absorbed. The amount of light that returns
to a PPG sensor’s photodetector is proportional to the
volume of blood in the tissue. The PPG signal represents
an average of all blood volume in the arteries, capillaries,
and any other tissue through which the light passed. The
PPG signal depends on the thickness and composition of the
tissue beneath the sensor and the position of the source and
receiver of the infrared light.
Most PPG sensors can be placed anywhere on the body,
from the earlobe to the vaginal wall, with the finger as the
most common location for recording a BVP signal. The PPG
sensor measures relative changes in the perfusion of the
blood through the tissue underneath the sensor. For example,
changes in the BVP signal can indicate relative changes in
the vascular bed due to vasodilation or vasoconstriction
(increase or decrease in blood perfusion) as well as changes
in the elasticity of the vascular walls, reflecting changes in
blood pressure. Because the blood volume in the arteries and
Biofeedback Ô Summer 2007
of time using measures of the volume of blood that passes
over a photoplethysmographic (PPG) sensor with each
pulse, also called blood volume pulse (BVP). Recently, HRV
training using BVP measurements has escaped out of the
laboratory and into the marketplace. BVP training devices
are commercially available as portable units such as the
StressEraser and the emWave or as units built for use with
computers such as the FreezeFramer or the Journey to Wild
Divine. By using these BVP training devices, individuals
can become aware of factors that increase or decrease their
HRV. For example, excessive sensory arousal; shallow, rapid
breathing; and excessive emotions of fear, worry, anger,
or panic will decrease HRV. On the other hand, reducing
sensory arousal, breathing slowly at approximately five to
seven breaths per minute, and experiencing emotions of
appreciation and love will increase HRV. In clinical settings,
practitioners use sophisticated multichannel biofeedback
systems to train clients to increase HRV and CRS. In most
clinical settings, heart rate information is derived from a
BVP signal rather than from an electrocardiography (ECG)
signal. Whereas the ECG signal is more precise (e.g., with
fewer movement artifacts), applying an ECG sensor is also
more cumbersome and obtrusive compared to applying a
BVP sensor (e.g., without gel or tape or placement on a chest).
Using PPG feedback devices alone and/or in conjunction
with respiratory feedback devices such as respiratory strain
gauges, clients can learn to increase HRV/RSA, as shown in
Figures 1 and 2.
The participant data from Figure 1 represent an average
respiration rate of 14 breaths per minute, with a corresponding
heart rate of 68 beats per minute (SD = 5.0 beats). The data
from Figure 2 represent an average respiration rate of 7
Figure 2. Blood volume pulse, blood volume amplitude, heart rate, and
respiration during training designed to increase cardiorespiratory synchrony.
55
Blood Volume Pulse
Figure 3. Heart rate is derived from measures of blood volume pulse by
measuring the interbeat interval and then transforming this information into
beats per minute. For example, the interbeat interval of 0.80 seconds is equal
to a heart rate of 75 beats per minute, whereas the interbeat interval of 0.93
seconds is equal to a heart rate of 64.5.
capillary bed increases with each arterial pulsation, heart
rate can be estimated from the BVP signal. Heart rate, or the
number of heartbeats per minute, is calculated by estimating
the time interval between the heartbeats, called the interbeat
interval. The time in seconds of the interbeat interval is
divided into 60 seconds to calculate the beat-by-beat heart
rate, as shown in Figure 3.
The time between each beat can vary; therefore, the
estimated beat-by-beat heart rate can also vary. There are
two estimates of the variability of the heart rate: variability
estimated by the SDANN or variability estimated by the
standard deviation of the average beat-by-beat heart rate
(SDAHR). A methodological issue includes the fact that
the raw BVP signal must be artifact free before meaning is
assigned to the SDANN and SDAHR measures of variability
in the heart rate data. The next section address artifacts in
the BVP signal.
Inspecting the Raw BVP Signal for Artifacts
Summer 2007 Ô Biofeedback
When analyzing average heart rates, it is important to
eliminate artifacts before calculating estimates of HRV. Heart
rate averages calculated from the data may not be reliable if
artifacts exist, as illustrated in the following vignette:
56
What happened? He reported increased arousal during
the stressful imagery, yet his heart rate was higher during
the initial baseline condition. It was only when I included
the raw blood volume pulse (BVP) signal from which the
heart rate was calculated that I realized the average signal
was incorrect.
Figure 4. Recording of the heart rate derived from the raw blood volume pulse
during the baseline period. The average heart rate included a segment of very
rapid heart beat reflecting movement artifact rather than a true increase in
average heart rate.
Figure 5. When movement artifact is excluded from the signal shown in Figure
4, the actual heart rate is estimated at 61.05 beats per minute.
This dialogue illustrates issues related to artifacts in the
BVP data. The data included movement artifacts, as shown in
Figure 4. After eliminating the movement artifact as shown
in Figure 5, the heart rate information was nearly five beats
lower. Note also that in this participant, there was a slight
increase in heart rate during the stressful imagery rehearsal
once the segments of data affected by artifact were removed.
Once movement artifacts are removed from BVP
estimates of heart rate, the BVP measure of HRV becomes
an easy-to-administer technology for use in clinical settings.
HRV is usually a sign of cardiac health, as it suggests that the
heart has flexibility in response to the demands of the body.
HRV reflects cardiovascular health as a sign of sympathetic
Peper, Harvey, Lin, Tylova, Moss
Figure 6. Example of blood volume pulse signal with amplitude and timing
markers. Time t1 (between markers 1 and 5) indicates the interbeat interval and
is used to calculate the heart rate. Pulse measure P1 (marker 1) is a measure
of pulse amplitude. Volume at V (marker 3) is the indicator of the blood volume
influenced by the dicrotic notch. Reprinted with permission from Hlimonenko,
Meigas, and Vahisalu (2003).
and parasympathetic nervous system balance. When HRV
is absent or reduced, it may signal pathology (Del Pozo et
al., 2004; Kleiger et al., 1987). Namely, when HRV is low, as
indicated by an SDANN <50 milliseconds and an SDAHR
<2.5 beats, there is a fourfold increase in relative risk of death
after myocardial infarction compared to those who have
a high HRV (SDANN >100 milliseconds and SDAHR >5
beats; Kleiger et al., 1987). Whereas artifact-free BVP signals
can be used for training of HRV, BVP measures may also be
used for estimating cardiovascular health in terms of blood
pressure because the BVP signal also reflects changes in the
elasticity of the vascular walls (Asada, Shaltis, Reisner, Rhee,
& Hutchinson, 2003; Babchenko et al., 2001; Speckenbach &
Gerber, 1999; Weng, Matz, Gehring, & Konecny, 2002).
Changes in BVP Waveform Shape Reflect
Other Types of Cardiovascular Health
Changes in BVP Amplitude May Reflect Moment-by-Moment Sympathetic/Parasympathetic and Cognitive/Emotional Activity
The BVP amplitude displays moment-by-moment HRV
and may offer significant insight into individual emotional
responses, as illustrated in Figure 8. Figure 8 shows
psychophysiological responses during a standardized stress
protocol. The participant’s responsiveness to internal and
Biofeedback Ô Summer 2007
The shape of the BVP waveform may be an indicator of
cardiovascular variables such as blood pressure because
stiffer arterial walls are associated with higher blood
pressure (Speckenbach & Gerber, 1999). The raw BVP signal
is the product of many factors that influence blood flow
through the vascular bed. The raw BVP wave pattern is the
result of the recording location, the heart’s left ventricular
ejection, and the elasticity/stiffness of the aorta and arteries.
The shape of BVP signal can be used for deriving interbeat
interval (t1), which in turn can be transformed into an
estimate of heart rate as well as the pulse amplitude (P1),
both of which represent the relative increase in blood volume
caused by the heart contracting, as shown in Figure 6. The
elasticity in the arterial vasculature is partially reflected
in the magnitude of the dicrotic notch signal, shown as
marker 3 in Figure 6. The dicrotic notch signal depends on
the interaction of the initial pressure wave when the heart
contracts, arterial stiffness that decreases the pulse transit
time, and the reflected pressure wave from the peripheral
arterial bed.
The loss of arterial wall elasticity is usually an indicator of
aging and suggests an increased risk of cardiovascular disease,
especially hypertension (Izzo & Shykoff, 2001). Increasing
arterial stiffness decreases the pulse transit time (PTT). PTT
can be time referenced to the R-wave of the ECG signal
corresponding time between P1 and V of the BVP signal
graph in Figure 6. The stiffer the arterial walls, the faster the
PTT, with the effect most pronounced in the periphery of
the toes compared to the fingers and ears (Allen & Murray,
2002). Decreases in PTT have been significantly correlated
with an increase in blood pressure and age. The decrease in
PTT affects the BVP waveform by appearing as a diminution
of the dicrotic notch as the initial and reflected pressure
waves come closer together. The relationship between the
PTT and BVP waveforms is illustrated by the BVP and the
blood pressure recordings of parents and children of varying
ages, as illustrated in Figure 7.
Even though HRV training offers great clinical potential,
it is important to remember that many factors affect blood
circulation and thereby the BVP signal. These include
sympathetic arousal, which induces vasoconstriction;
decreased sympathetic arousal and increased parasympathetic arousal, which induce vasodilation especially as the
person relaxes; low external temperature, which induces
vasoconstriction; recreational and prescription drugs (e.g.,
alcohol increases vasodilation, whereas nicotine increases
vasoconstriction); and illnesses such as Raynaud’s disease,
which is characterized by arteriole vasoconstriction and
reduced blood flow in the fingers. Changes in the BVP signal
can be very rapid and thereby may reflect sudden shifts in
arousal or cognitions. Because arousal and cognitions change
rapidly, the BVP signal may also be used as an indicator of
transient processes.
57
Blood Volume Pulse
Figure 7. Comparison of finger blood volume pulse recording of parents (62year-old father and 52-year-old mother) and child (17-year-old daughter). The
mother has borderline hypertension. The absence of the dicrotic notch in
the borderline hypertensive (top) tracing suggests a stiffening of the arteries,
indicating increased blood pressure.
Figure 8. Example blood volume pulse amplitude analysis by measuring the
percentage change for each condition [(maximum – minimum)/maximum ×
100].
Summer 2007 Ô Biofeedback
This exploration of emotions and BVP training could include
some of the following:
58
external physical and emotional stressors is vividly depicted
in the variations of BVP amplitude presented Figure 8. The
pattern portrays decreases in amplitude in the BVP signal in
response to prompts such as sighs and claps that triggered
sympathetic activation. In this participant, eye closure during
the protocol evoked an unanticipated and large decrease
in the BVP amplitude compared to any of the physical or
imagined stress conditions. This unanticipated decrease in
BVP may be interpreted as a kind of anticipatory anxiety.
Initially, the eye closure was only an instruction before
asking the person to think about a stressful experience. The
idiosyncratic BVP amplitude changes are a window into her
psychological processes. The large response (vasoconstriction)
to the instruction “close your eyes” indicates the participant’s
general ongoing arousal and vigilance. Instead of relaxing
to the “close your eyes” instruction, she reported later that
she started to think, “What will happen now?” She was
continuously checking if her experience of the world was
safe. This cognitive reaction is often found in people who
are anxious, fearful, or want to please others. The BVP
amplitude can also indicate the extent to which the person
has been captured by his or her emotions; the more captured,
the longer it takes for the BVP signal to return to some
baseline level. In the example above of anticipatory anxiety,
the BVP signal returned rapidly to baseline, reflecting a
state of vigilance rather than the activation of a memory
of an emotional trauma. By observing her response pattern
during the training session, the participant could explore
strategies that would enable her to feel safe and to prevent
a decrease in her BVP amplitude when she closes her eyes.
• Identification of the thoughts, feelings, and emotions
associated with the responses
• Exploration of past and present patterns that let the
person interpret the world as unsafe
• Exploration of how to reframe the experience so that the
vigilance response is not evoked
• Practice of physiological desensitization so that the
response is extinguished
The BVP signal may also be interpreted in terms of
peripheral temperature changes because the signal reflects
changes in vasoconstriction and vasodilation.
The Use of BVP Amplitude as an Indicator
of Changes in Peripheral Temperature
Changes in BVP represent changes in the blood volume. If
BVP amplitude increases, it reflects an increase in vasodilation,
which leads to an increase in peripheral temperature; if the
BVP amplitude decreases, it reflects a decrease in peripheral
circulation and a decrease in peripheral temperature. BVP
amplitude changes are very rapid and precede changes in
temperature, as temperature is a slow-averaging signal, as
shown in Figure 9.
When BVP is monitored simultaneously with temperature, the rapid BVP amplitude changes can facilitate
peripheral warming. For example, when the BVP amplitude
started to increase, even though the peripheral temperature
was still decreasing, it may help the clinician and participant
to identify emotional and cognitive images and sensations
Peper, Harvey, Lin, Tylova, Moss
Figure 9. Simultaneous recording of right and left blood volume pulse
(BVP), temperature, and electromyography signals. Temperature changes lag
behind the BVP amplitude changes. Even as BVP amplitude is increasing, the
temperature is still decreasing. From Zoe Talbot and Sarah Langensiepen,
personal communication.
that facilitate peripheral warming. Among these strategies
are slower and diaphragmatic breathing, autogenic phrases,
relaxation instructions, and imagery to increase peripheral
warmth. In addition to BVP signals being used as indicators of
peripheral temperature, the BVP signal may also be used for
treating headaches as well as for monitoring sexual arousal.
Overlooked BVP Applications: Treatment
of Migraine and Monitoring Human Sexual
Arousal
and temporal BVP reduction biofeedback in the treatment
of migraine. Results showed that temporal constriction
and finger temperature biofeedback were equally effective
in controlling migraine headaches and produced greater
benefits than the waiting list condition. Nestoriuc and
Martin (2007) performed a meta-analysis examining the
efficacy of biofeedback training in treating migraine. Results
showed that biofeedback training was more effective than
control conditions. The strongest improvements were in the
frequency of migraine attacks and perceived self-efficacy.
BVP feedback yielded higher effect sizes than peripheral
skin temperature feedback and electromyography feedback.
Moderator analyses revealed biofeedback training in
combination with home training to be more effective than
therapies without home training.
BVP has also been used as an indicator of sexual arousal.
For example, PPG sensors measure tissue engorgement by
blood from any location ranging from the nasal septum to
measure cerebral blood flow to the vaginal wall to measure
sexual arousal. As early as 1967, Palti and Bercovici used
a PPG sensor to measure vasoengorgement of the vaginal
wall as an indicator of sexual arousal to erotic stimuli, as
shown in Figure 10 (Brotto, Basson, & Gorzalka, 2004; Palti
& Berovici, 1967). Other applications of BVP measurement
and feedback have yet to be fully explored
Exploring Other Uses of the BVP Signal
The biofeedback applications of BVP are expanding rapidly
with the availability of the new economic HRV/CRS home
trainers. These portable and relatively inexpensive devices
allow people to become aware of factors that affect the BVP
signal. Through HRV training, participants can develop
Biofeedback Ô Summer 2007
Using BVP feedback to encourage vasomotor control offers
numerous clinical applications such as the treatment for
migraine by recording the BVP from the temporal artery
(Feuerstein & Adams, 1977). Allen and Mills (1982) used
photoelectric plethysmograph feedback to train to selfregulate BVP amplitude in eight female migraine sufferers.
Participants learned to increase and decrease BVP amplitude
on the scalp at superficial temporal artery (STA) and finger
locations. The results of the research showed a significant
relationship between voluntary pulse amplitude changes in
the BVP amplitude measured at the STA and corresponding
pain reports during a migraine. Hoelscher and Lichstein
(1983) used a temporal BVP biofeedback protocol in treating
chronic cluster headache patients. The result shows a 70%
reduction in daily headache frequency and a 45% decrease
in headache severity. Improvement was maintained at 1, 3,
6, 12, and 21 months of follow-up. Large decreases in the
consumption of migraine abortives, narcotic analgesics, and
antiemetics were also observed. Gauthier, Lacroix, Coté,
Doyon, and Drolet (1985) reported using finger warming
Figure 10. The vaginal blood volume and pulse amplitude of a woman who is
shown a neutral stimulus and an erotic stimulus. The genital response occurs
within seconds of the presentation of an erotic stimulus in sexually healthy
women, regardless of age or menopausal status. Reprinted with permission
from Brotto and Gorzalka (2006).
59
Blood Volume Pulse
sympathetic/parasympathetic balance and master strategies
to prevent and reverse illness. In addition, easily available
computerized monitoring providing the corresponding
digital analysis such as power spectrum analysis (fast Fourier
transform) and feedback may allow new areas of biofeedback
BVP applications to emerge. Among those are the following:
• Analysis and training of specific components of the BVP
signal, such as increasing the dicrotic notch to improve
cardiovascular flexibility or reducing high blood pressure.
• Correlating BVP waveforms recorded from multiple
locations on the body with Chinese pulse used as a
diagnostic tool in traditional Chinese medicine (TCM).
In TCM, for more than 2000 years, a stiff pulse has been
an indicator of aging and/or pathology.
• Exploring the changes in blood flow as indicators of
illness or health by recording the BVP response patterns
simultaneously from multiple locations such as from
different sympathetically enervated dermatomes.
• Ongoing analysis and treatment of male and female
sexual dysfunctions as well as development of strategies
to enhance orgasmic quality. This could possibly include
correlations of penile and vaginal wall engorgement with
peripheral blood flow changes in other areas of the body
that may contribute to sexual arousal.
• Combining BVP monitoring with psychotherapy, as the
rapid changes in BVP amplitude may indicate cognitive
and emotional responses.
• Teaching increasing BVP amplitude to improve circulation
and thus regeneration in cases of diabetic ulcers and
frostbites (Rice & Schindler, 1992; Graul, Stanculescu,
Peper, Johansen, & Doyle, 2004).
The goal of this article was to raise awareness of the
use of BVP technologies beyond the calculation of HRV or
RSA/CRS indicators. Whereas other uses of the PPG/BVP
technology will emerge in the future, those described here
offer a good start for stimulating new areas of research.
Summer 2007 Ô Biofeedback
Acknowledgments
60
This article was adapted from E. Peper, H. Tylova, K. H.
Gibney, and R. Harvey, Mastery Through Experience: A
Self-Teaching Laboratory Manual for Biofeedback and SelfRegulation skills (unpublished manuscript). We thank Fred
Shaffer for his insightful feedback.
References
Allen, J., & Murray. A. (2002). Age-related changes in
peripheral pulse timing characteristics at the ears, fingers
and toes. Journal of Human Hypertension, 16, 711–717.
Allen, R. A., & Mills, G. K. (1982). The effects of unilateral
plethysmographic feedback of temporal artery activity
during migraine head pain. Journal of Psychosomatic
Research, 26, 133–140.
Asada, H. H., Shaltis, P., Reisner, A., Rhee, S., & Hutchinson,
R. C. (2003). Mobile monitoring with wearable
photoplethysmographic biosensors. IEEE English Medical
Biology Magazine, 22(3), 28–40.
Babchenko, A., Davidson, E., Ginosar, Y., Kurz, V., Faib,
I., Adler, D., et al. (2001). Photoplethysmographic
measurement of changes in total and pulsatile tissue blood
volume, following sympathetic blockade. Physiological
Measurement, 22, 389–396.
Brotto, L. A., Basson, R., & Gorzalka, B. B. (2004).
Psychophysiological assessment in premenopausal
sexual arousal disorder. Journal of Sexual Medicine, 1,
266–277.
Brotto, L. A., & Gorzalka, B. B. (2006). The vaginal
photoplethysmograph. Retrieved November 10,
2006, from http://www.psych.ubc.ca/~bglab/equipment.
html
Del Pozo, J. M., Gevirtz, R. N., Scher, B., & Guarneri, E. (2004).
Biofeedback treatment increases heart rate variability in
patients with known coronary artery disease. American
Heart Journal, 147(3), G1–G6.
Feuerstein, M., & Adams, H. E. (1977). Cephalic vasomotor
feedback in the modification of migraine headache.
Applied Psychophysiology and Biofeedback, 2, 214–
254.
Gauthier, J., Lacroix, R., Coté, A., Doyon, J., & Drolet, M.
(1985). Biofeedback control of migraine headaches: A
comparison of two approaches. Applied Psychophysiology
and Biofeedback, 10, 139–159.
Gevirtz, R. N. (2003). The promise of HRV biofeedback:
Some preliminary results and speculations. Biofeedback,
31(3), 18–19.
Giardino, N. D., Chan, L., & Borson, S. (2004). Combined
heart rate variability and pulse oximetry biofeedback
for chronic obstructive pulmonary disease. Applied
Psychophysiology and Biofeedback, 29, 121–133.
Graul, M., Stanculescu, A., Peper, E., Johansen, K. L., &
Doyle, J. W. (2004). Possible treatment of diabetic ulcer
for a kidney dialysis patient: A case report [Abstract].
Applied Psychophysiology and Biofeedback, 29, 299.
Hlimonenko, I., Meigas, K., & Vahisalu, R. (2003). Waveform
analysis of peripheral pulse wave detected in the fingertip with photoplethysmograph. Measurement Science
Review, 3(2), 49–52.
Hoelscher, T. J., & Lichstein, K. L . (1983). Blood volume
pulse biofeedback treatment of chronic cluster headache.
Biofeedback and Self-Regulation, 8, 533–541.
Izzo, J. L., & Shykoff, B. E. (2001). Arterial stiffness:
Clinical relevance, measurement, and treatment. Review
Cardiovascular Medicine, 2(1), 29–34, 37–40.