File

Jolly Thermodynamics
Significance of binary mixture concepts and its applications
JOLLY THERMODYNAMIC’S TEAM - April 9, 2015
R e l a t e d C o n c e p t s
P h a s e R u l e
R a o u l t ’ s L a w
A n t o i n e ’ s E q u a t i o n
M a s s B a l a n c e
I d e a l M i x t u r e
BINARY MIXTURE-ELEMENTAL APPLICATION APPROACH 1
Introduction
First of all, a binary system is the mixture of two any components, where they can be at equilibrium or not. But the aim of this article is to explain the importance of the equilibrium inside of a mixture in order to understand how useful is to master this discipline or at least the concept. Therefore, in the real world exists the chemical industry where most of its initial products have something to do with extracting anything from a natural resource, for example: petroleum from earth, essences from flowers, sugars from trees and so on. But in the end there is always a common denominator, that in this case is the separation process from A to B, which in general a good example of this could be distillation or flash (fig.1.) for a first approach to a further complex operations.
Nevertheless, it has always to be remember the need of two phase to make any separation and these phases should be in equilibrium.
Fig. 1. Scheme of a Toluene/Benzene flash process
In the other hand, the diagram shows the a unit operation called it, “Flash”. Where it exhibit two components, in this case Benzene and Toluene at F flow. But it could have been any other components. The nomenclature for this kind of process is always similar at this one, where the input flow is represented by F, and if there is another input, this would be respectively F1, F2, F3 until Fn ends. This also works for the output lines such as the vapor ones and the liquid ones, which are named with the letter V and L as you see on the figure 1. Here after, it can say that T stands for a temperature in Celsius and P for pressure in atmospheres and don't forget that de liquid extract is also knows as bottom extract. In
BINARY MIXTURE-ELEMENTAL APPLICATION APPROACH 2
the upper flow, the vapor is known as overheat or refinate. So now on, it can formulate a problem. For example 1: Given the composition of F 35% benzene and 65% toluene at certain Temperature ( 100 ºC )and Pressure( 1 atm) , which would be the composition after a flash operation of its outcomes flows as it appears on figure 1.
Fig.2. Boundaries of the system
The first thing is to define the boundaries(fig. 2). Maybe, this looks silly for just one process but whenever your working with a connected process, it helps a lot. Second, it needs to be specify what are the tools to solve the question. In this case, they are asking us to determine de composition of Vapor (V) and Liquid (L) given at certain temperature and pressure. So it suppose to be in equilibrium this equipment unless they tell us to not. But it’s not posible to not be in equilibrium, otherwise it couldn't make any separation. Think about it. ( you can ask us from contact at the webpages).
Secondly, it need to be defined the tools to resolve this problem. In this case we need to know how many variables are needed in order to solve it and you can know this with the Gibbs’ phase rule. Then, it need to be calculated the the saturation pressure for a further operations with Raoult’s law and temperature with Antoine’s equation to define with is the most volatile component to do the T-xy or P-xy diagram ( it will be explained this topic later, don't worry) . Afterward, the use of Raoult’s law will tell us the composition of Vapor and liquid, however before it used this law, it’s an obligation to do the mass balance before.
Let's get started according to the above information!!!
BINARY MIXTURE-ELEMENTAL APPLICATION APPROACH 3
Procedure
Step 1- Phase rule this rule tell us the grades of freedom in a closed equilibrium system for example to this case
F = C - P + N
where F stands for degrees of freedom ( the conditions of the system)
C stands for the number of components
P stands for number of separation phases
N stands for number of no compositional variables such temp, but for Gibbs’ rule is alway 2 because the equilibrium thermodynamic to delta Gibbs (change) is equivalent to a chemical potential in any phase for every component, namely
N=2.
So the final equation is:
F= 2 - 2 + 2 = 2
C stands for the number of components which are benzene and toluene, so it equals 2.
P stands for number of separation phases which it says a mixture benzene/toluene over a flash operation, so it is talking about liquid (1) -vapor (1) , so it equal 2.
N (already explained)
F = 2 ( the simply math).
The interpretation is that it needs variables to be specify two intensive variables to describe a a phase system and on this problem the pressure and temperature are known.
( Read the problem again or look on figure 1).
***Intensive variables = intensive properties= properties that doesn't depend on the the size of the system, for example temperature and pressure.
BINARY MIXTURE-ELEMENTAL APPLICATION APPROACH 4
Step 2 - Antoine’s equation
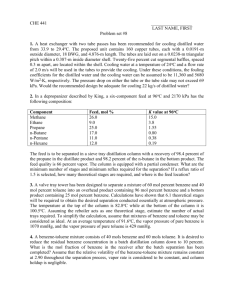
The saturation pressure and temperature with Antoine’s Law.
ln P i sat
= A
B
−
T + C
A, B and C are Antoine’s constants and the values can be found on books tables such as Elementary Principles of Chemical Processes from Richard Mark Felder. ( you can ask for the book sending a email to any of the team members or click on the link below).
( be careful with the unit, most of the times is used mmHg for pressure and centigrade for temperature)
T is the temperature of the system for toluene:
Saturation pressure of toluenes is 556.3432 mmHg = .73220 atm
BINARY MIXTURE-ELEMENTAL APPLICATION APPROACH 5
for benzene: saturation pressure of benzene is 1350.1089 mmHg=1.7764
For temperature it needs to be solve Antoine’s equation for T. with the same constante and the the pressure of the system, once you have the temperature. They need to be compare to other one and the one with a lower boiling point will be the most volatile component, this helps you to understand what component tends to be in liquid or vapor given certain conditions of T and P.
In this case for Benzene the temperature is 78.298 ºC and for toluene is 100.012 ºC. So
Benzene is most volatile component. This is used until it will calculate the dew point and bubble point for TXY diagram of PXY diagram. ( it will revised later on).
Step 3- Raoult’s law for ideal solutions
The Raoult’s equation tells you what is the amount that goes as liquid for every component of any mixture ( in this case a binary one) and what is the amount of benzene and toluene on the liquid outcome of a flash operation.
= Total pressure of the system in equilibrium
= composition of the vapor phase, component (Benzene)
= composition of the liquid phase, component 1 (Toluene)
= pressure vapor , component 1 as binary mixture it knows the next one: (FIRST PAIR OF EQUATIONS)
X benzene
+ X toluene
= 1 ( where X is the liquid phase)
Y benzene
+ Y toluene
= 1 ( where Y is the vapor phase) and the Raoult’s equation stay like this:
BINARY MIXTURE-ELEMENTAL APPLICATION APPROACH 6
(SECOND PAIR OF EQUATIONS)
Benzene
PY
1
=P
1
Sat X
1
Toluene
PY
1
=P
1
Sat X
1 for easy writing it will be using 1 for benzene and 2 for toluene. In order to avoid rewriting both words in the next operations. It seeks to know Y and X for toluene.benzene respectively.
BINARY MIXTURE-ELEMENTAL APPLICATION APPROACH 7
The results are the number underlined.
Now, it knows the composition but given a that F was 100 mol/s. The requirement is to calculate the mol fraction for L and for V knowing the bottom extract and overheat composition of both components.
Step 4- Mass Balance and Caudal/flow Calculation
Global Balance
F= V + L -> 100 = F +L
Component Balance
Benzene -> 100*(.35) = 0.44V + 0.25 L or
Toluene -> 100*(.65) = 0.54V +0.74 L
It solves for V either the Bezene's or Toluene’s equation with the global balance equation.
Finally, it gets the flow of liquid (L) , which is 47.3684 mol/s and for vapor ( V) is
52.6316 mol/s.
All this work was for and ideal system in order to do the same from a non-ideality the requisite is the use of state equations and in Raoult’s law part, it changes to the henry
BINARY MIXTURE-ELEMENTAL APPLICATION APPROACH 8
constants, that are also found in tables books or journal. This is explained in other articles of our webpage.
Step 5- Conclusion
This articles sets the basis of and ideal flash operation of a mixture. which the importance is to understand that knowing this you can change a little bit the algorithm to known a multicomponent mixtures that can be found in nature. The interest for example is the purification and separation of ethanol from blue agave to produce tequila or any other product of your interest.
Step 6- Looking forward
The binary mixture information of the example above can be used to learn how to use PXY diagrams and TXY diagrams
Do you remember the data?
If you say no, it’s ok. We will tell you again, don't worry
Now it shows how to graph a PXY diagram in Microsoft Excel, but first the reason to do that is because sometimes with charts and graphs is easy to see some properties of the system. That’s why you do this, and it is also a form to simplify the information in just one piece. Other application to this is the see the line of dew and bubble point. Remember there is a lot ways to do this, but in here will be with Microsoft excel and then in ASPEN simulator.
Remember that it was a Benzene Toluene mixture with 35% of the benzene at 100ºC and 1 atms, the Antione's constants and Saturation Temperature and Pressure were the right below:
BINARY MIXTURE-ELEMENTAL APPLICATION APPROACH 9
The unique new operation was the conversion of arms to kpa, because it will graph at this units just because we like it that way :).
Knowing the all the data to make the graph, you should understand what you want to do is know each point between the nature of your two components and overtime you use more points your graph will be more accurate. So understanding this and that you want to graph in Y axis the pressure versus in the X axis the “x” and “y” composition you should be varying the pressure between the lower value and the higher value of your system which are the saturation pressure that you calculate before. the one in the Black box with red letter if you don’t remember and then recalculate the composition of your
“x” and “y” of the your components. But remember that you will graph the most volatile component.
The equations for “x” and “y” is the Raoult one.
x
1
=
P
P
1 sat
−
−
P
2 sat
P
2 sat y
1
= x
1
*
P
P
1 sat
So you are looking to make the table below…
Once you have it, you should first go to insert graph in excel and select scatter and in select data you will choose firs the blue and and the yellow column, which are the pressure that you are varying and composition of the liquid of the benzene. The you will add a new serie in the same graph and then you will graph the blue column versus the green one, which are the the vapor composition of the benzene in the system. You may not
BINARY MIXTURE-ELEMENTAL APPLICATION APPROACH 10
graph the toluene composition but if you notice knowing one composition in a binary mixture tells you implicit the composition of the other component, that’s why.
Nevertheless this can only do with binary or even tertiary mixture, because for multicomponent mixtures you can not graph them. Finally the graph is below.
Step 7- Simulation “the easiest way”
In some cases the use of some softwares will help you to do things faster and in this with ASPEN simulator you can obtain this graph very easy in lees than 5 minutes as long as the data base has the require components of your mixture. Supposing that, the way to do it is the next one: first of all you need aspen, open and select-new project or new file second select-blank simulation third select-add new component and in the new window you will look for your componentes, add benzene.
Fourth. look for toluene-add
So now you should have something like the image below.
BINARY MIXTURE-ELEMENTAL APPLICATION APPROACH 11
And you will go to your upper corner and select binary and then click on
Run analysis the green square and that’s it. Your will get your TXY diagram or PXY diagram.
BINARY MIXTURE-ELEMENTAL APPLICATION APPROACH 12
You can learn more of these in the section of theoretical plates.
BINARY MIXTURE-ELEMENTAL APPLICATION APPROACH 13