PAINÒ 143 (2009) 123–129
www.elsevier.com/locate/pain
Psychological determinants of problematic outcomes following Total
Knee Arthroplasty
Michael Sullivan a,*, Michael Tanzer b, William Stanish c, Michel Fallaha d, Francis J. Keefe e,
Maureen Simmonds f, Michael Dunbar c
a
Department of Psychology, McGill University, 1205 Docteur Penfield, Montréal, Que., Canada H3A 1B1
Department of Surgery, McGill University, Montréal, Qc, Canada
c
Department of Surgery, Dalhousie University, Halifax, NS B3H 3A7, Canada
d
Department of Surgery, Université de Montréal, Montréal, Qc H1T 2M4, Canada
e
Duke Pain Prevention and Treatment Research Program, Duke University, Durham, NC 27710, USA
f
School of Physical and Occupational Therapy, McGill University, Montréal, Qc H3G 1Y5, Canada
b
a r t i c l e
i n f o
Article history:
Received 30 July 2008
Received in revised form 6 January 2009
Accepted 17 February 2009
Keywords:
Arthritis
TKA
Pain catastrophizing
Fear of movement
Depression
Surgical outcomes
Pain
a b s t r a c t
The primary objective of the present study was to examine the role of pain-related psychological factors
in predicting pain and disability following Total Knee Arthroplasty (TKA). The study sample consisted of
75 (46 women, 29 men) individuals with osteoarthritis of the knee who were scheduled for TKA. Measures of pain severity, pain catastrophizing, depression, and pain-related fears of movement were completed prior to surgery. Participants completed measures of pain severity and self-reported disability
6 weeks following surgery. Consistent with previous research, cross-sectional analyses revealed significant correlations among measures of pre-surgical pain severity, pain catastrophizing, depression and
pain-related fears of movement. Prospective analyses revealed that pre-surgical pain severity and pain
catastrophizing were unique predictors of post-surgical pain severity (6-week follow-up). Pain-related
fears of movement were predictors of post-surgical functional difficulties in univariate analyses, but
not when controlling for pre-surgical co-morbidities (e.g. back pain). The results of this study add to a
growing literature highlighting the prognostic value of psychological variables in the prediction of
post-surgical health outcomes. The results support the view that the psychological determinants of
post-surgical pain severity differ from the psychological determinants of post-surgical disability. The
results suggest that interventions designed to specifically target pain-related psychological risk factors
might improve post-surgical outcomes.
Ó 2009 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
1. Introduction
Arthritis is the leading cause of disability in North America.
Osteoarthritis (OA) is the most common form of arthritis, affecting
approximately 21 million people in the United States and three
million people in Canada. Patients with severe OA of the knee
who experience significant pain and pain-related disability, or joint
deformity may be considered candidates for Total Knee Arthroplasty (TKA) [13,49,55,63].
TKA improves function and yields significant pain relief for the
majority of patients who undergo the procedure [20,36,60]. There
are indications, however, that 15–30% of patients may continue to
experience significant pain following surgery [2,18,36,43,54,
57,62]. The number of patients reporting ongoing symptoms of
pain decreases through the first year post-surgery, yet as many
* Corresponding author. Tel.: +1 514 398 5677; fax: +1 514 343 4896.
E-mail address: michael.sullivan@mcgill.ca (M. Sullivan).
as 20% of patients report moderate to severe pain one year postsurgery [2,31]. Studies have shown that, for many patients, pain
persists following TKA, in spite of objective indicators of surgical
success [8,31,45].
Considerable research has accumulated indicating that medical
status variables alone cannot fully account for symptoms of pain
and disability associated with OA [33,39–41]. Biopsychosocial
models have been put forward suggesting that a complete understanding of pain-related outcomes will require consideration of
physical, psychological and social factors [40,77,81]. Research has
supported the view that psychological factors play a significant
role in the experience of pain and disability associated with arthritis [33,37,41,42].
In other domains of research, variables such as pain catastrophizing, pain-related fears of movement, and depression have been
identified as risk factors for prolonged pain and disability
[50,67,82]. High levels of pain catastrophizing predict
ongoing pain, and more severe disability in individuals with
0304-3959/$36.00 Ó 2009 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
doi:10.1016/j.pain.2009.02.011
124
M. Sullivan et al. / PAINÒ 143 (2009) 123–129
musculoskeletal conditions [10,23,51,70]. Pain catastrophizing has
also been shown to predict post-surgical pain [29,58]. Pain-related
fears of movement have been shown to be significant determinants
of disability associated with low back pain. Depressive symptoms
have also been implicated as factors contributing to the transition
from acute to chronic pain [14,19,65,73].
Pain catastrophizing, pain-related fears of movement, and
depressive symptoms may be overlapping or unique risk factors
for problematic TKA outcomes. To date, research has yet to simultaneously examine the role of pain catastrophizing, pain-related fears
of movement and depressive symptoms as risk factors for problematic recovery following TKA. From a clinical perspective, such research is important because it could help identify which of these
factors should be key targets for psychosocial interventions designed to improve TKA outcomes. From a theoretical perspective,
the predictive value of biopsychosocial conceptualizations will increase as research begins to address more directly how different
model-relevant constructs summate or interact to give rise to
pain-related outcomes.
In the present study, patients scheduled for TKA were assessed
one week prior to surgery and then again 6-weeks post-surgery.
Analyses examined the value of pre-surgical measures of pain
catastrophizing, pain-related fears of movement and depressive
symptoms in the prediction of post-surgical pain severity and
physical function.
2. Methods
2.2.3. Pain catastrophizing
The Pain Catastrophizing Scale [PCS; 72] consists of 13 items
describing different thoughts and feelings that individuals may
experience when they are in pain. The PCS yields a total score and
subscale scores for rumination, magnification and helplessness.
The PCS total and PCS subscales have been shown to have good to
high internal consistency (Cronbach’s [17] alphas: total = .87, rumination = .87, magnification = .66, helplessness = .78), and to be
associated with heightened pain, and disability [68–70,72]. In the
present study, Cronbach’s [17] alphas were .94 (PCS Total), .88
(rumination), .83 (magnification), and .89 (helplessness).
2.2.4. Pain-related fears of movement
The Tampa Scale for Kinesiophobia [TSK; 47] is a 17-item questionnaire that assesses pain-related fear of movement. The TSK has
been shown to be internally reliable (coefficient alpha = .77)
[46,80]. The TSK has been associated with various indices of behavioural avoidance and disability in patients with a variety of health
conditions including OA [16,59,70].
2.2.5. Depressive symptoms
The Patient Health Questionnaire-9 (PHQ-9) was used as a measure of depressive symptom severity. The PHQ-9 is a 9-item questionnaire that asks respondents to indicate the frequency with
which they experience each of the nine symptoms considered in
the diagnostic criteria for Major Depression [66]. The PHQ-9 has
been shown to be a valid and reliable measure of depressive symptoms in patients with a variety of medical conditions [27,34,48].
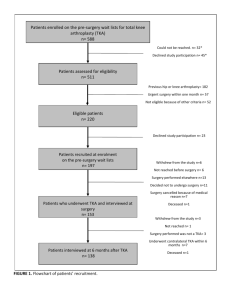
2.1. Participants
The study sample consisted of 75 (46 women, 29 men) individuals who had been scheduled for TKA at one of three hospitals in
Eastern Canada. The mean age of the sample was 68.6 years with
a range of 48 to 91 years. The majority of the samples were married
(85%) and had completed at least 12 years of education (90%).
Forty-five patients had TKA of the right knee and 30 had TKA of
the left knee.
2.2. Measures
2.2.1. Pain and function
The Western Ontario and McMaster University Osteoarthritis
Index (WOMAC) was used to assess clinical health status relevant
to TKA outcomes [4]. The WOMAC is a self-administered instrument that yields a total score and subscale scores for (1) Pain, (2)
Stiffness, and (3) Physical Function. For the purposes of the present
study, only the subscale scores for pain and physical function are
reported. Scores on the Pain and Function subscales were converted to a 0–100 scale to facilitate cross-scale and cross-study
comparison [6,9]. Higher scores reflect worse pain and function.
Since the conversion to 100-point scales simply involves multiplication by a constant, the psychometric properties of the instrument are not affected. The WOMAC has been shown to be a valid
and reliable index of health functioning associated with OA, and
has been shown to be sensitive to changes in function subsequent
to TKA [3–5].
2.2.2. Co-morbidities
Common co-morbid conditions that can influence TKA outcomes include hypertension, osteoarthrosis of other joints, diabetes mellitus, chronic obstructive pulmonary disease (COPD), and
history of tobacco use. Co-morbidity was assessed with the Charlson Co-morbidity Index [CCI; 15]. On this measure, respondents
are asked to indicate the presence and severity of 13 different
health conditions. The number of different health conditions endorsed by the respondent is summed to yield a total score [15].
2.2.6. Analgesic consumption
A variety of analgesics were used for pain control post-operatively. Two separate indices of 48-h analgesic consumption were
derived: total NSAID consumption (aspirin equivalent units) and
total opioid consumption (morphine equivalent units). For NSAIDS,
the following conversions were applied: aspirin 650 mg = acetominophen 650 mg = ibuprofen 200 mg = naproxen 125 mg [30]. For
opioids, the following conversions were applied: IV morphine
1 mg = oxycodone 1.5 mg = hydromorphone .15 mg; PO morphine
6 mg = oxycodone 3 mg = hydromorphone .75 mg [1].
3. Procedure
Patients who were scheduled for TKA at one of three collaborating hospitals were invited to participate in the research. Patients
were informed that the research was concerned with the physical,
psychological and social determinants of recovery following surgery. They were invited to sign a consent form as a condition of
participation. Participants received $25 as compensation for completing the questionnaires. The research was approved by the
Research Ethics Boards of the McGill University Health Centre,
the Hôpital Maisonneuve-Rosemont and the Capital Health
Authority of Nova Scotia. Participants completed questionnaires
at the time of their pre-surgical evaluation (one day prior to surgery), and at the time of their 6-week post-surgical follow-up.
Surgeries were performed by seven surgeons from three
different hospitals. Surgery was performed under spinal (n = 44)
or general anesthesia (n = 31). Thirty-two patients also received a
femoral block.
The same approach to surgery was used by all surgeons. A midline incision with para-medial arthrotomy was performed. Intramedullary alignment guides were used for femoral and tibial cuts.
The posterior cruciate ligament was left or resected at the discretion of the surgeon. Bicondylar femoral and tibial components
were implanted and cemented. Resurfacing of the patellae was at
the discretion of the surgeon. A polyethylene liner was inserted between the metallic femoral and tibial prostheses. Post-operatively,
M. Sullivan et al. / PAINÒ 143 (2009) 123–129
patients were given systemic prophylactic antibiotics and prophylactic anticoagulant to decrease deep venous thrombosis risk.
Anterior–posterior (AP) and lateral knee radiographs were taken
and reviewed before patient was transferred to a floor for continued care. The radiographs were reviewed to ensure that the prosthesis was inserted properly and that alignment was correct.
Compression dressings were removed the day after surgery and
continued passive range of motion was applied. Patients were instructed to weight bear as tolerated.
At 6-week follow-up, radiographic analysis indicated that all
implants were in good mechanical alignment (2–7° of femoral-tibial valgus) with satisfactory implant placement. None of the study
patients had evidence of ligament instability on follow-up examination. By clinical standards, all patients in the study sample were
considered surgical successes.
3.1. Data analytic approach
Initial analyses addressed whether scores on predictor variables
or outcome variables varied as a function of surgeon or hospital.
No significant differences were found. As such, surgeon and hospital
are not included in the predictive analyses. No significant differences
on outcome measures were found as a function of type of anesthesia.
One patient developed infection post-surgery. Analyses were conducted with and without this patient. The pattern of results was
not affected and the patient was retained in the predictive analyses.
Descriptive statistics were computed on sample characteristics
and questionnaire scores. Pearson correlations were used to assess
the concurrent and prospective relations among study variables.
Bonferroni correction was used to control for alpha inflation. Multiple regression analyses were used to assess the value of pre-surgical psychological variables in predicting post-surgical pain and
function. In predictive analyses, initial scores on measures of pain
and function were used as covariates. In the regression results reported, all tolerance coefficients were greater than .60 such that no
problem of multicollinearity was indicated.
4. Results
4.1. Sample characteristics
As shown in Table 1, age distribution, BMI and WOMAC pain
and function scores were similar to those reported in previous reTable 1
Sample characteristics.
Age
BMI
Comorbid
Surgery duration
48 h Aspirin eq
48 h Morphine eq
Pain Pre
Function Pre
Pain Post
Function Post
PCS
TSK
PHQ-9
Women
Men
p
Total
68.1 (10.2)
30.5 (5.7)
2.4 (1.2)
95.5 (12.2)
529.27 (2433.8)
44.4 (19.0)
60.9 (13.9)
66.0 (12.1)
44.1 (14.0)
44.2 (13.7)
15.4 (12.5)
29.2 (9.1)
5.6 (4.4)
69.5 (9.2)
29.0 (4.5)
2.5 (1.2)
101.3 (16.1)
5226.6 (2165.7)
42.0 (16.7)
58.5 (13.7)
64.2 (10.6)
50.8 (18.1)
46.4 (12.9)
18.0 (11.0)
28.5 (7.0)
4.2 (4.3)
ns
ns
ns
ns
ns
ns
ns
ns
ns
ns
ns
ns
ns
68.6 (9.6)
29.7 (5.2)
2.4 (1.2)
97.7 (14.0)
5267.1 (2319.0)
43.4 (18.1)
59.9 (13.7)
65.1 (11.6)
46.6 (15.8)
45.0 (13.3)
16.2 (11.9)
29.0 (8.3)
5.0 (4.4)
Note: N = 75. BMI = body mass index; Comorbid = number of comorbid health
conditions; 48 h Aspirin eq = analgesic consumption first 48 hours post-op, aspirin
equivalent mgs; 48 h Morphine eq = analgesic consumption first 48 hours post-op,
morphine equivalent mgs; Pain Pre = WOMAC Pain Score, pre-surgery; Function
Pre = WOMAC Physical Function Score, pre-surgery; Pain Post = WOMAC Pain Score,
post-surgery; Function Post = WOMAC Physical Function Score, post-surgery;
PCS = Pain Catastrophizing Scale; TSK = Tampa Scale for Kinesiophobia; PHQ9 = Patient Health Questionnaire-9.
125
search on patients undergoing TKA [7,56]. The average patient reported having two co-morbid health conditions. The most
frequently reported co-morbid health conditions were high blood
pressure (71%), back pain (50%), and heart disease (15%). The presence or absence of high blood pressure and heart disease did not
influence scores on post-operative pain and function. The presence
of back pain was associated with more impaired post-operative
function, t(73) = 2.0, p < .05, but was unrelated to post-operative
knee pain.
PCS scores were higher than those that have been reported in
previous studies of patients undergoing TKA [64]. The distribution
of depression scores suggests that the average patient was experiencing mild symptoms of depression. According to the suggested
cut-off for clinically meaningful depressive symptomatology (e.g.,
PHQ-9 > 15), 4/75 patients scored in the moderate-severe range
of depression.
As expected, there were significant decreases in pain ( 13.3),
t(74) = 7.2, p < .001, and functional difficulties ( 20.0), t(74) =
11.7, p < .001 from the pre-surgical to post-surgical evaluation.
4.2. Concurrent relations among psychological variables, pain and
function: pre-surgery
Table 2 presents the pre-surgical concurrent relations between
psychological variables and health status variables. In order to control for alpha inflation, Bonferonni corrected alpha was set at .005.
Consistent with previous research in individuals with arthritic
conditions, measures of pain catastrophizing, pain-related fears,
and depression were significantly correlated with measures of pain
and function [33,38,42,64]. In addition to the correlations presented in Table 2, age was inversely correlated with pre-surgical
pain, r = .38, p < .01, and BMI, r = .46, p < .01.
4.3. Prospective relations between pre-surgical psychological variables
and post-surgical outcomes
Table 3 presents the correlations between pre-surgical psychological measures and post-surgical measures of pain and function.
Bonferonni corrected alpha was set at .005. Pre-surgical scores on
measures of pain catastrophizing, pain-related fears, and depression were significantly correlated with post-surgical measures of
pain and function. Of the three PCS subscales, the magnification
subscale showed the highest correlations with post-surgical pain
and function. As expected, post-surgical measures of pain and
function were also significantly correlated with pre-surgical measures of pain and function. BMI, number of co-morbidities, dura-
Table 2
Concurrent relations among pre-surgical variables.
1.
2.
3.
4.
5.
6.
7.
8.
Pain Pre
Function Pre
TSK
PHQ-9
PCS tot
PCS rumin
PCS magni
PCS helps
1
2
3
4
5
6
7
.67**
.32**
.48**
.44**
.40**
.40**
.42**
.37**
.49**
.49**
.40**
.44*
.55**
.44**
.50**
.46**
.46**
.58**
.45**
.36**
.43**
.53**
.90**
.88**
.96**
.69**
.78**
.82**
Note: N = 75. Pain Pre = WOMAC Pain Score, pre-surgery; Function Pre = WOMAC
Physical Function Score, pre-surgery; Pain Post = WOMAC Pain Score, post-surgery;
Function Post = WOMAC Physical Function Score, post-surgery; TSK = Tampa Scale
for Kinesiophobia; PHQ-9 = Patient Health Questionnaire-9; PCS tot = Pain Catastrophizing Scale Total Score; PCS rumin = rumination subscale of the PCS; PCS
magni = magnification subscale of the PCS; PCS helps = helplessness subscale of the
PCS.
**
p < .005.
M. Sullivan et al. / PAINÒ 143 (2009) 123–129
126
Table 3
Prospective relations between pre-surgical and post-surgical variables.
Pain Post
**
Pain Pre
Function Pre
PCS tot
PCS rumin
PCS magni
PCS helps
TSK
PHQ-9
BMI
Co-morbidities
Surgery duration (min)
48 h Aspirin
48 h Morphine
.43
.30
.46**
.38**
.47**
.42**
.31
.26
.05
.11
.05
.13
.06
Table 5
Regression analysis predicting post-surgical physical function (WOMAC function).
Function Post
**
.37
.29
.32
.17
.37**
.35**
.38**
.24
.16
.05
.04
.08
.01
Note: N = 75. Pain Pre = WOMAC Pain Score, pre-surgery; Function Pre = WOMAC
Physical Function Score, pre-surgery; Pain Post = WOMAC Pain Score, post-surgery;
Function Post = WOMAC Physical Function Score, post-surgery; PCS tot = Pain
Catastrophizing Scale Total Score; PCS rumin = rumination subscale of the PCS; PCS
magni = magnification subscale of the PCS; PCS helps = helplessness subscale of the
PCS; TSK = Tampa Scale for Kinesiophobia; PHQ-9 = Patient Health Questionnaire-9;
48 h Aspirin eq = analgesic consumption first 48 hours post-op, aspirin equivalent
mgs; 48 h Morphine eq = analgesic consumption first 48 hours post-op, morphine
equivalent mgs.
**
p < .005.
tion of surgery, and post-surgical analgesic intake were not significantly correlated with post-operative pain and function.
4.4. Predicting post-surgical pain and function
Separate hierarchical regressions were computed to assess the
value of pre-surgical measures of catastrophizing, pain-related
fears, and depression in the prediction of post-surgical pain and
function. In each regression, age and sex were entered in the first
step. In the second step of the analyses, the pre-surgical score on
the dependent variable (i.e. pain or function) was entered. In the
third step of the analyses, co-morbid back pain (absent, present)
was entered. Finally, the three psychological variables were
entered.
As shown in Table 4, age and sex were entered in the first step
of the analysis but the contribution of this step failed to attain statistical significance (p = .07). However, in the final regression equation, sex did emerge as a significant predictor of post-surgical pain
where women reported more intense pain than men. Pre-surgical
pain accounted for 17% of the variance in post-surgical pain, before
entering the psychological variables. Co-morbid back pain did not
contribute to the prediction of post-operative knee pain. The PCS,
Table 4
Regression analysis predicting post-surgical pain severity (WOMAC pain).
Step 1
Age
Sex
Step 2
Pain Pre
Step 3
Back pain
Step 4
PCS
TSK
PHQ-9
Beta
R2change
.01
.20*
.06
2.3 (2, 72)
.28
.17
16.5 (1, 71)**
.11
.01
.7 (1, 70)
.28*
.07
.01
.08
2.8 (3, 67)*
*
Fchange
Note: N = 75. Pain Pre = WOMAC Pain Score, pre-surgery; PCS = Pain Catastrophizing
Scale; TSK = Tampa Scale for Kinesiophobia; PHQ-9 = Patient Health Questionnaire9. Values in parentheses are degrees of freedom. Beta weights are from the final
regression equation.
*
p < .05.
**
p < .01.
Step 1
Age
Sex
Step 2
Function Pre
Step 3
Back pain
Step 4
PCS
TSK
PHQ-9
Beta
R2change
Fchange
.09
.09
.02
1.0 (2, 72)
.13
.07
6.1 (1, 71)**
.23*
.05
4.2 (1, 70)*
.12
.24 .04
.09
2.5 (3, 68) Note: N = 75. Function Pre = WOMAC Physical Function Score, pre-surgery;
PCS = Pain Catastrophizing Scale; TSK = Tampa Scale for Kinesiophobia; PHQ9 = Patient Health Questionnaire-9. Values in parentheses are degrees of freedom.
Beta weights are from the final regression equation.
p = .06.
*
p < .05.
**
p < .01.
TSK and PHQ-9 were entered in the final step of the regression
and contributed significant variance to the prediction of post-surgical pain beyond the variance accounted for by age, sex and presurgical knee and back pain. Examination of the beta weights from
the final regression equation indicated that, of the three psychological variables, only the PCS (beta = .28, p < .05) contributed significant unique variance to the prediction of post-surgical pain.
Controlling for all other variables in the regression equation, sex,
pre-surgical knee pain and pain catastrophizing accounted for 4%,
8% and 8% of the variance in post-surgical pain, respectively.
Table 5 shows the results of the regression analysis for the prediction of post-surgical function. Pre-surgical WOMAC function
scores accounted for 7% of the variance in post-surgical WOMAC
function scores. Co-morbid back pain accounted for 5% of the variance in post-surgical WOMAC function scores. The three psychological variables were entered in the final step of the analysis but
failed to contribute significantly to the prediction of post-surgical
WOMAC function scores. Examination of the beta weights for the
final regression equation revealed that the predictive value of the
TSK was marginally significant, p = .06.
5. Discussion
The present findings add to a growing body of literature suggesting that certain psychological variables might predispose individuals to adverse pain-related outcomes following surgical
intervention. The results of the present research are consistent
with previous studies showing that higher levels of pre-surgical
pain and functional difficulties predict post-surgical pain and functional difficulties [26,31]. The results are also consistent with
previous research showing that women experience more intense
post-surgical pain than men [25,61]. The present research extends
previous findings in showing that pain catastrophizing predicts
post-surgical pain to the same degree as pre-surgical pain. Furthermore, the contribution of pain catastrophizing to the prediction of
post-surgical pain was independent of the contribution of pre-surgical pain. Pain-related fears made a near-zero contribution to the
prediction of post-surgical pain. Pain-related fears of movement
were found to predict post-surgical functional difficulties following
TKA in univariate but not multivariate analyses.
From a theoretical perspective, the findings are consistent with
cognitive behavioural models of pain that suggest that negative
appraisals (e.g., catastrophic thinking) of pain will influence the
intensity and persistence of pain experience. Pain catastrophizing
has been construed as a multidimensional construct comprising
M. Sullivan et al. / PAINÒ 143 (2009) 123–129
elements of rumination (i.e. excessive focus on pain sensations),
magnification (i.e. heightened perception of the threat value of
pain symptoms) and helplessness (i.e., beliefs that the control of
pain is beyond one’s ability) [75]. In previous research, each of
these dimensions has been shown to have an adverse impact on
pain experience [11,21,79].
In addition to the psychological processes that link catastrophizing to adverse pain outcomes, there are indications that catastrophizing might also influence neurophysiological processes
involved in pain modulation. High levels of pain catastrophizing
have been associated with poorer response to opioids for both clinical and experimental pain [24,32,35]. It has been suggested that
pain catastrophizing might interfere with descending pain-inhibitory systems and might facilitate neuroplastic changes in the
spinal cord during repeated painful stimulation, subsequently promoting sensitization in the CNS [28].
The findings are also partially consistent with fear-avoidance
models that have been developed to account for the development
of pain-related disability. According to fear-avoidance models,
pain-related fears will lead to activity avoidance or escape, and
in turn, contribute to functional disability. Prolonged periods of
inactivity then compound disability through deconditioning and
heightened risk of developing co-morbidities (e.g., obesity and diabetes) [33].
The results of the present research highlight the importance of
examining psychological predictors of post-surgical outcomes
from a multivariate perspective. Like previous research, the present study revealed significant variance overlap among measures
of catastrophic thinking, pain-related fears and depressive symptoms (20–25% shared variance). In spite of this degree of shared
variance, the findings suggest that pain catastrophizing and painrelated fears of movement are differentially associated with postsurgical pain and function. Not only do these findings support
the conceptual distinctiveness of pain catastrophizing and pain-related fears of movement, but they also have implications for the
nature of interventions that might be considered for individuals
scheduled for TKA.
Many individuals with OA will continue to experience debilitating symptoms of pain following surgery. In the present study, at
6 weeks post-surgery, 30% of patients reported their pain at rest
to be moderate or severe. Research suggests that approximately
half of these patients might go on to develop chronic pain conditions [31,43,44]. Once symptoms of pain become chronic, available
methods of managing pain, whether pharmacological or psychological, have only modest impact on suffering and function. Persistent pain symptoms have been identified as central determinants
of disability, both pre- and post-TKA [26,52,78]. Persistent pain
symptoms following TKA can contribute to a trajectory increasing
distress and disability associated with discontinuation of life role
activities, progressive decline toward a sedentary lifestyle, and social isolation [12,39].
If individuals at risk for post-surgical pain and disability can be
identified before the problem becomes chronic, individual’s suffering might be prevented or reduced to a significant degree. The results of the present study suggest that measures of pain
catastrophizing and pain-related fears of movement might be considered as screening measures for identifying patients at risk for
problematic outcomes following TKA. Individuals identified at risk
might then be considered for targeted interventions that might
prevent the development of persistent pain and disability following surgery.
Research suggests that pain catastrophizing and pain-related
fears of movement can best be construed as modifiable risk factors
for problematic health outcomes. Although pain catastrophizing
and pain-related fears of movement have been shown to be stable
in the absence of intervention, both variables have been shown to
127
decrease in response to intervention [71,74]. Although comparative studies have yet to be conducted, there are data to suggest that
cognitive techniques might be most effective in reducing pain
catastrophizing while behavioural (e.g., exposure) techniques
might be most effective in reducing pain-related fears of movement [76,83]. The present research suggests that interventions that
specifically target pain catastrophizing and pain-related fears of
movement could be adapted for patients scheduled for TKA, and
hold promise of reducing the degree to which patients will continue to experience significant pain and disability post-surgery.
To date, preventative psychosocial interventions for OA patients
have primarily taken the form of broad-based educational programs. Since education programs do not select individuals at risk,
nor do they target specific prognostic factors for pain and disability, it is not surprising that they have yielded only modest impact
on surgical outcomes [53].
There have been previous reports suggesting that depression
might contribute to poor TKA outcomes, defined either in terms
of pain, function or quality of life indices [25,26]. For example, Faller et al. [22] reported that pre-operative depression scores predicted post-operative scores of a measure of functional
difficulties at 3 months and 12 months following TKA. In the latter
study, the unique contributions of pain catastrophizing and painrelated fears of movement were not simultaneously assessed. In
the present study, depression was associated with pain and function scores in univariate analyses (but not when correlations were
Bonferonni corrected), and not in the multivariate analyses.
Although the negative impact of depression needs to be considered
in the treatment of all debilitating health conditions, depression
might not make a unique contribution to problematic health outcomes following TKA.
Roth et al. [64] recently reported the results of a study examining predictors of post-surgical pain in a sample of patients having
undergone TKA. Cross-sectional analyses revealed significant relations between pain intensity and pain catastrophizing, but prospective analyses failed to show significant predictive value of
pre-operative pain or pre-operative pain catastrophizing. A number of factors might account for these inconsistent findings. First,
the level of catastrophizing of patients in the Roth et al. sample
(Total PCS = 4.7) was considerably lower than that in the present
study (Total PCS = 16.2). It is possible that high scores on pain
catastrophizing were insufficiently represented in the Roth et al.
study to reveal a prospective relation with post-surgical outcomes.
Roth et al. also assessed pain in the first three days following TKA
while the present study assessed TKA outcomes at 6-weeks postsurgery. It is possible that due to higher medication intake during
the days immediately following surgery, relations between pain
catastrophizing and pain intensity might have been obscured.
More research is needed to specify the conditions that determine
whether or not prospective relations between psychological variables and post-surgical outcomes will be observed.
Caution must be exercised in the interpretation of the present
findings. It is important to consider that cross-study discrepancies
provide grounds for questioning the reliability or robustness of the
findings. Sample sizes have been modest in most studies conducted to date on the psychological predictors of post-surgical outcomes. Confidence in the reliability of the findings awaits
replication with a larger sample size. In addition, the present sample was followed up for only 6 weeks. While the findings point to
the influence of catastrophic thinking and pain-related fears of
movement on pain and function at 6 weeks post-surgery, it is unclear whether these relations would persist over a longer period of
time. In many studies of orthopedic surgery outcomes, follow-up
periods might be as long as two years. Since psychological factors
likely have a dynamic relation with changing health status over
time, longer follow-up periods might be needed to provide a
128
M. Sullivan et al. / PAINÒ 143 (2009) 123–129
clearer portrait of the manner, and the time frame within which
pre-surgical psychological variables might impact on orthopedic
post-surgical outcomes.
In spite of these limitations, the results of the present study
showed that pre-surgical levels of pain catastrophizing and painrelated fears of movement predicted post-surgical pain and functional difficulties, respectively. Research has highlighted that the
prevalence of problematic outcomes following TKA is alarmingly
high, in spite of indicators of surgical success. The findings of the
present study suggest that psychological variables might be playing an important role as determinants of problematic health outcomes following TKA. These findings might be important to
consider in implementing screening procedures to identify individuals at risk for problematic outcomes and for developing risk-factor targeted interventions aimed at preventing problematic
outcomes following TKA.
Conflict of interest
None of the authors has any financial interests in the findings of
the present study.
Acknowledgements
This research was supported by a grant from the Canadian Institutes of Health Research (CIHR). The authors thank Karen Smith,
Donalda Dickey, Allan Hennigar, Kory Arsenault and Anne-Marie
Laliberté for their assistance in participant recruitment and data
collection.
References
[1] Arnold R, Weissman DE. Calculating opioid dose conversions #36. J Palliat Med
2003;6:619–20.
[2] Baker PN, van der Meulen JH, Lewsey J, Gregg PJ. The role of pain and function
in determining patient satisfaction after total knee replacement. Data from the
National Joint Registry for England and Wales. J Bone Joint Surg Br
2007;89:893–900.
[3] Bellamy N. Pain assessment in osteoarthritis: experience with the WOMAC
osteoarthritis index. Semin Arthritis Rheum 1989;18:14–7.
[4] Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation
study of WOMAC: a health status instrument for measuring clinically
important patient relevant outcomes to antirheumatic drug therapy in
patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–40.
[5] Bellamy N, Kean WF, Buchanan WW, Gerecz-Simon E, Campbell J. Double blind
randomized controlled trial of sodium meclofenamate (Meclomen) and
diclofenac sodium (Voltaren): post validation reapplication of the WOMAC
Osteoarthritis Index. J Rheumatol 1992;19:153–9.
[6] Bishoff-Ferrari HA, Lingard EA, Losina E, Baron JA, Roos EM, Phillips CB,
Mahomed NN, Barrett J, Katz JN. Psychosocial and geriatric correlates of
functional status after total hip replacement. Arthritis Rheum 2008;15:390–5.
[7] Boonstra MC, De Waal Malefijt MC, Verdonschot N. How to quantify knee
function after total knee arthroplasty. The Knee, in press.
[8] Brander VA, Stulberg SD, Adams AD, Harden RN, Bruehl S, Stanos SP, Houle T.
Predicting total knee replacement pain: a prospective, observational study.
Clin Orthop Relat Res 2003;20:27–36.
[9] Bullens PHJ, van Loon CJM, De Waal Malefijt MC, Laan RFJ, Veth RPH. Patient
satisfaction after total knee arthroplasty. J Athroplasty 2001;16:740–7.
[10] Burton AK, Tillotson KM, Main CJ, Hollis S. Psychosocial predictors of outcome
in acute and subchronic low back trouble. Spine 1995;20:722–8.
[11] Bushnell MC, Villemure C, Duncan GH. Psychophysical and neurophysiological
studies of pain modulation by attention. In: Price DD, Bushnell MC, eds.
Psychological methods of pain control: basic science and clinical
perspectives2004. Seattle (WA): IASP Press.
[12] Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a
biopsychosocial perspective. Biol Psychiatry 2003;54:399–409.
[13] Canadian Joint Replacement Registry. CJRR report: total hip and total knee
replacements in Canada 2004. In: Information CIfH, ed; 2004. p. 91.
[14] Carroll LJ, Cassidy JD, Cote P. Depression as a risk factor for onset of an episode
of troublesome neck and low back pain. Pain 2004;107:134–9.
[15] Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying
prognostic comorbidity in longitudinal studies: development and validation. J
Chronic Dis 1987;40:373–83.
[16] Crombez G, Vlaeyen JW, Heuts PH, Lysens R. Pain-related fear is more disabling
than pain itself: evidence on the role of pain-related fear in chronic back pain
disability. Pain 1999;80:329–39.
[17] Cronbach LJ. Coefficient alpha and the internal structure of tests.
Psychometrika 1951;16:297–334.
[18] Dickstein R, Heffes Y, Shabtai EI, Markowitz E. Total knee arthroplasty in the
elderly: patients’ self-appraisal 6 and 12 months postoperatively. Gerontology
1998;44:204–10.
[19] Druss B, Rosenbeck R, Sledge W. Health and disability costs of depressive
illness in a major US corporation. Am J Psychiatry 2000;157:1274–8.
[20] Dunbar MJ. Subjective outcomes after knee arthroplasty. Acta Orthop Scand
Suppl 2001;72:1–63.
[21] Edwards RR, Bingham 3rd CO, Bathon J, Haythornthwaite JA. Catastrophizing
and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis
Rheum 2006;55:325–32.
[22] Faller H, Kirschner S, Konig A. Psychological distress predicts functional
outcomes at three and twelve months after total knee arthroplasty. Gen Hosp
Psychiatry 2003;25.
[23] Feuerstein M, Huang GD, Haufler AJ, Miller JK. Development of a screen for
predicting clinical outcomes in patients with work-related upper extremity
disorders. J Occup Environ Med 2000;42:749–61.
[24] Fillingim RB, Hastie BA, Ness TJ, Glover TL, Campbell CM, Staud R. Sex-related
psychological predictors of baseline pain perception and analgesic responses
to pentazocine. Biol Psychol 2005;69:97–112.
[25] Fisher DA, Dierckman B, Watts MR, Davis K. Looks good but feels bad: factors
that contribute to poor results after total knee arthroplasty. J Athroplasty
2007;22:39–42.
[26] Fortin PR, Clarke AE, Joseph L, Liang MH, Tanzer M, Ferland D, Phillips C,
Partridge AJ, Belisle P, Fossel AH, Mahomed N, Sledge CB, Katz JN. Outcomes of
total hip and knee replacement: preoperative functional status predicts
outcomes at six months after surgery. Arthritis Rheum 1999;42:1722–8.
[27] Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical
settings with the Patient Health Questionnaire (PHQ): a diagnostic metaanalysis. J Gen Intern Med 2007;22:1596–602.
[28] Goodin BR, McGuire L, Allshouse M, Stapleton L, Haythornthwaite JA, Burns N,
Mayes LA, Edwards RR. Associations between catastrophizing and endogenous
pain-inhibitory processes: sex differences. J Pain 2009;10:180–90.
[29] Granot M, Ferber SG. The roles of pain catastrophizing and anxiety in the
prediction of postoperative pain intensity: a prospective study. Clin J Pain
2005;21:439–45.
[30] Habib S, Matthews RW, Scully C, Levers BG, Shepherd JP. A study of the
comparative efficacy of four common analgesics in the control of postsurgical
dental pain. Oral Surg Oral Med Oral Pathol 1990;70:559–63.
[31] Harden RN, Bruehl S, Stanos S, Brander V, Chung OY, Saltz S, Adams A, Stulberg
SD. Prospective examination of pain-related and psychological predictors of
CRPS-like phenomena following total knee arthroplasty: a preliminary study.
Pain 2003;106:393–400.
[32] Haythornthwaite J, Clark M, Pappagallo M, Raja S. Pain coping strategies play a
role in the persistence of pain in post-herpetic neuralgia. Pain
2003;106:453–60.
[33] Heuts PH, Vlaeyen JW, Roelofs J, de Bie RA, Aretz K, van Weel C, van Schayck
OC. Pain-related fear and daily functioning in patients with osteoarthritis. Pain
2004;110:228–35.
[34] Huang FY, Chung H, Kroenke K, Delucchi KL, Spitzer RL. Using the Patient
Health Questionnaire-9 to measure depression among racially and ethnically
diverse primary care patients. J Gen Intern Med 2006;21:547–52.
[35] Jacobsen PB, Butler RW. Relation of cognitive coping and catastrophizing to
acute pain and analgesic use following breast cancer surgery. J Behav Med
1996;19:17–29.
[36] Jones CA, Voaklander DC, Johnston DW, Suarez-Almazor ME. Health related
quality of life outcomes after total hip and knee arthroplasties in a community
based population. J Rheumatol 2000;27:1745–52.
[37] Keefe FJ, Bonk V. Psychosocial assessment of pain in patients having rheumatic
diseases. Rheum Dis Clin North Am 1999;25:81–103.
[38] Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid
arthritis pain: catastrophizing as a maladaptive strategy. Pain 1989;37:51–6.
[39] Keefe FJ, Caldwell DS, Martinez S, Nunley J, Beckham J, Williams DA. Analyzing
pain in rheumatoid arthritis patients. Pain coping strategies in patients who
have had knee replacement surgery. Pain 1991;46:153–60.
[40] Keefe FJ, France C. Pain: biopsychological mechanisms and management. Am
Psychol Soc 1999:137–41.
[41] Keefe FJ, Lefebvre JC, Egert JR, Affleck G, Sullivan MJ, Caldwell DS. The
relationship of gender to pain, pain behavior, and disability in osteoarthritis
patients: the role of catastrophizing. Pain 2000;87:325–34.
[42] Keefe FJ, Smith SJ, Buffington AL, Gibson J, Studts JL, Caldwell DS. Recent
advances and future directions in the biopsychosocial assessment and
treatment of arthritis. J Consult Clin Psychol 2002;70:640–55.
[43] Kennedy DM, Hanna SE, Stratford PW, Wessel J, Gollish JD. Preoperative
function and gender predict pattern of functional recovery after hip and knee
arthroplasty. J Arthroplasty 2006;21:559–66.
[44] Kennedy DM, Stratford PW, Hanna SE, Wessel J, Gollish JD. Modeling early
recovery of physical function following hip and knee arthroplasty. BMC
Musculoskelet Disord 2006;7:100.
[45] Kim TK, Chang CB, Kang YG, Kim SJ, Seong SC. Causes and predictors of
patient’s dissatisfaction after uncomplicated total knee arthroplasty. J
Arthroplasty 2009;42:263–71.
[46] Klenerman L, Slade PD, Stanley IM, Pennie B, Reilly JP, Atchison LE, Troup JD,
Rose MJ. The prediction of chronicity in patients with an acute attack of low
back pain in a general practice setting. Spine 1995;20:478–84.
M. Sullivan et al. / PAINÒ 143 (2009) 123–129
[47] Kori SH, Miller RP, Todd DD. Kinesiophobia: a new view of chronic pain
behavior. Pain Manag 1990;47:35–43.
[48] Li C, Friedman B, Conwell Y, Fiscella K. Validity of the Patient Health
Questionnaire 2 (PHQ-2) in identifying major depression in older people. J
Am Geriatr Soc 2007;55:596–602.
[49] Liang MH, Cullen KE, Larson MG, Thompson MS, Schwartz JA, Fossel AH,
Roberts WN, Sledge CB. Cost-effectiveness of total joint arthroplasty in
osteoarthritis. Arthritis Rheum 1986;29:937–43.
[50] Linton SJ. New avenues for the prevention of chronic musculoskeletal pain and
disability. Amsterdam: Elsevier; 2002.
[51] Linton SJ. Environment and learning factors in the development of chronic
back pain and disability. Seattle (WA): IASP Press; 2004.
[52] McAlindon T, Cooper C, Dieppe P. Determinants of disability in osteoarthritis of
the knee. Ann Rheumatid Dis 1993;52:258–62.
[53] McDonald S, Hetrick S, Green G. Pre-operative education for hip or knee
replacement. Cochrane Database of Systematic Reviews 2004, Issue 1. Art. No.:
CD003526. DOI 10.1002/14651858.CD003526.pub2.
[54] Murray DW, Frost SJ. Pain in the assessment of total knee replacement. J Bone
Joint Surg 1998;80:426–31.
[55] Norman-Taylor FH, Palmer CR, Villar RN. Quality-of-life improvement
compared after hip and knee replacement. J Bone Joint Surg Br 1996;78:74–7.
[56] Nunez M, Nunez E, del Val JL, Ortega R, Segur JM, Hernandez MV, Lozano L,
Sastre S, Macule F. Health-related quality of life in patients with osteoarthritis
after total knee replacement: factors influencing outcomes at 36 months of
follow-up. Osteoarthritis Cartilage 2007;15:1001–7.
[57] Parker JC, Bradley LA, DeVellis RM, Gerber LH, Holman HR, Keefe FJ, Lawrence
TS, Liang MH, Lorig KR, Nicassio PM, Revenson TA, Rogers MP, Wallston KA,
Wilson MG, Wolfe F. Biopsychosocial contributions to the management of
arthritis disability Blueprints from an NIDRR-sponsored conference. Arthritis
Rheum 1993;36:885–9.
[58] Pavlin DJ, Sullivan MJ, Freund PR, Roesen K. Catastrophizing: a risk factor for
postsurgical pain. Clin J Pain 2005;21:83–90.
[59] Picavet HS, Vlaeyen JW, Schouten JS. Pain catastrophizing and kinesiophobia:
predictors of chronic low back pain. Am J Epidemiol 2002;156:1028–34.
[60] Ritter MA, Albohm MJ, Keating EM, Faris PM, Meding JB. Comparative
outcomes of total joint arthroplasty. J Arthroplasty 1995;10:737–41.
[61] Ritter MA, Wing JT, Berend ME, Davis KE, Meding JB. The clinical effect of
gender on outcome of total knee arthroplasty. J Athroplasty 2008;23:331–6.
[62] Robertsson O, Dunbar M, Pehrsson T, Knutson K, Lidgren L. Patient satisfaction
after knee arthroplasty: a report on 27, 372 knees operated on between 1981
and 1995 in Sweden. Acta Orthop Scand 2000;71:262–7.
[63] Robertsson O, Dunbar MJ, Knutson K, Lidgren L. Past incidence and future
demand for knee arthroplasty in Sweden: a report from the Swedish Knee
Arthroplasty Register regarding the effect of past and future population
changes on the number of arthroplasties performed. Acta Orthop Scand
2000;71:376–80.
[64] Roth ML, Tripp DA, Harrison MH, Sullivan M, Carson P. Demographic and
psychosocial predictors of acute perioperative pain for total knee arthroplasty.
Pain Res Manag 2007;12:185–94.
129
[65] Rush AJ, Polatin P, Gatchel RJ. Depression and chronic low back pain:
establishing priorities in treatment. Spine 2000;25:2566–71.
[66] Spitzer RL, Williams JBW, Kroenke K, et al. Patient Health Questionnaire-9.
Pfizer Inc., Prime MD Today; 1999.
[67] Sullivan M, Feuerstein M, Gatchel RJ, Linton SJ, Pransky G. Integrating
psychological and behavioral interventions to achieve optimal rehabilitation
outcomes. J Occup Rehabil 2005;15:475–89.
[68] Sullivan MJ, Neish NR. Catastrophizing, anxiety and pain during dental hygiene
treatment. Community Dent Oral Epidemiol 1998;26:344–9.
[69] Sullivan MJ, Stanish W, Sullivan ME, Tripp D. Differential predictors of pain
and disability in patients with whiplash injuries. Pain Res Manag
2002;7:68–74.
[70] Sullivan MJ, Stanish WD. Psychologically based occupational rehabilitation:
the Pain-Disability Prevention Program. Clin J Pain 2003;19:97–104.
[71] Sullivan MJL, Adams H, Rhodenizer T, Stanish WD. A psychosocial risk factortargeted intervention for the prevention of chronic pain and disability
following whiplash injury. Phys Ther 2006;86:8–18.
[72] Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development
and validation. Psychol Assess 1995;7:524–32.
[73] Sullivan MJL, Reesor K, Mikail S, Fisher R. The treatment of depression in
chronic low back pain: review and recommendations. Pain 1992;50:5–13.
[74] Sullivan MJL, Stanish WD. Psychologically based occupational rehabilitation:
the Pain-Disability Prevention Program. Clin J Pain 2003;19:97–104.
[75] Sullivan MJL, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA,
Lefebvre JC. Theoretical perspectives on the relation between catastrophizing
and pain. Clin J Pain 2001;17:52–64.
[76] Thorn BE, Pence LB, Ward LC, Kilgo G, Clements KL, Cross TH, Davis AM, Tsui
PW. A randomized clinical trial of targeted cognitive behavioral treatment to
reduce catastrophizing in chronic headache sufferers. J Pain 2007;8:938–49.
[77] Turk DC. Biopsychosocial perspective on chronic pain. New York: Guilford;
1996.
[78] van Baar ME, Dekker J, Lemmens JA, Oostendorp RA, Bijlsma JW. Pain and
disability in patients with osteoarthritis of hip or knee: the relationship with
articular, kinesiological, and psychological characteristics. J Rheumatol
1998;25:125–33.
[79] Vangronsveld K, Van Damme S, Peters M, Vlaeyen J, Goossens M, Crombez G.
An experimental investigation on attentional interference by threatening
fixations of the neck in patients with chronic whiplash syndrome. Pain
2007;127:121–8.
[80] Vlaeyen JW, Kole-Snijders AM, Boeren RG, van Eek H. Fear of movement/
(re)injury in chronic low back pain and its relation to behavioral performance.
Pain 1995;62:363–72.
[81] Waddell G. The back pain revolution. Edinburg: Churchill Livingstone; 1998.
[82] Waddell G, Burton A, Main C. Screening to identify people at risk of longterm incapacity for work. London, UK: Royal Society of Medicine Press;
2003.
[83] Woods MP, Asmundson GJ. Evaluating the efficacy of graded in vivo exposure
for the treatment of fear in patients with chronic back pain: a randomized
controlled clinical trial. Pain 2008;136:271–80.