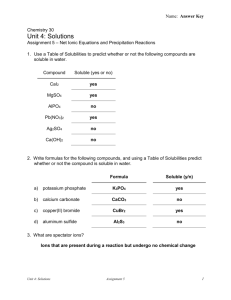
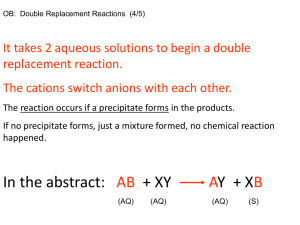
Instruction for Separation and identification of metal cations for
advertisement

Instrukcje do ćwiczeń on-line dla Studentów kierunku Environmental Protection and Management z przedmiotu Chemistry współfinansowane ze środków Unii Europejskiej w ramach Europejskiego Funduszu Społecznego, Program Operacyjny Kapitał Ludzki, nr umowy UDA-POKL 04.01.02.-00-137/1100 „Absolwent Wydziału Chemicznego Politechniki Gdańskiej – inżynier z przyszłością”. Korekta językowa:........................... Instruction for Separation and identification of metal cations for Environmental Protection and Management Agnieszka Pladzyk Gdaosk University of Technology, Chemical Faculty Department of Inorganic Chemistry 2013 1 TABLE OF CONTENTS I. General information…….……………………………………………………………………………………….……………………..3 II. General description of analytical groups………………………………………………………………………………………4 III. Exercise 1: Separation of I Group cations (Ag+, Pb2+, Hg22+)…………………………………………………..……...5 IV. Exercise 2: Separation of II Group cations (Hg2+, Bi3+, Cu2+, Cd2+)………………………………………...……….8 V. Exercise 3: Separation of III Group cations (Co2+, Ni2+, Fe3+, Mn2+, Cr3+, Al3+, Zn2+)……………………….12 VI. Exercise 4: Separation of IV-V Group cations (Ca2+, Ba2+, Mg2+, K+, Na+, NH4+)……………………….......16 2 I. General Information This manual is meant for students participating in the course of Inorganic Chemistry laboratory. The main purpose of this laboratory is to provide the Students an appreciation for the characterizations of chosen inorganic cations. During the first lab the Instructor will present the laboratory and important safety rules. During this laboratory the Student will be working individually. Every next four labs the Student will be writing the short test and analyzing the given sample that contains chosen cations from individual groups. At the end of every lab Student will write the report and will obtain the points. Lastly a final report is required and should include a complete description of the separation and identification of the Groups I to V metal contents found in the given samples. Include balanced reactions and significant observations. This is going to be an individual report from the whole work done during this lab. The overall grade from laboratory performance will be calculated by taking the sum of the points from all exercises and written tests. The final grade from the lab will depend on the final report given to instructor within two weeks from the last exercise. Exercises planned for realization during this laboratory will explore some of the strategies used for separation and identification of metal cations. The flow chart shown for each exercise is a classic method for separating complex mixtures of metal cations. Its central strategy is to separate the individual cations and to identify them. The major steps of those flow charts are explained in each exercise. 3 II. General description of analytical groups For the purpose of systematic qualitative analysis, cations are classified into five groups on the basis of their behavior against some reagent called GROUP REAGENTS. Classification is based on whether a cation reacts with these reagents with the formation of the precipitate or not. Group I Cations of this group form precipitates with dilute hydrochloric acid. Ions of this group are lead Pb2+, mercury(I) Hg22+ and silver Ag+. Group II The cations of this group are divided into IIA and IIB groups. IIA contains mercury(II) Hg2+, copper Cu2+, bismuth Bi3+, cadmium Cd2+ and lead Pb2+. The presence of lead cations in groups I and IIA is caused by partial solubility of PbCl2 in diluted hydrochloric acid and for that reason lead ions, if present in the sample, are not completely precipitated with Group I and are carried over into Group IIA. The IIB group contains arsenic As3+, antimony Sb3+ and tin Sn2+. We will not test for the Pb2+ and Group IIB cations in the qualitative analysis of Group II cations. Group III The cations of this group do not react either with diluted hydrochloric acid, or with hydrogen sulfide in diluted mineral acid medium. However they form precipitates with ammonium sulfide in neutral or ammoniac medium. Cations of this group are: cobalt Co2+, nickel Ni2+, iron(II) Fe2+, iron(III) Fe3+, chromium(III) Cr3+, manganese(II) Mn2+, aluminum Al3+ and zinc Zn2+. Group IV The cations of this group do not react with the reagents of Groups I, II and III. They form precipitates with ammonium carbonate in the presence of ammonium chloride and ammonia. Cations of this group are: calcium Ca2+, strontium Sr2+ and barium Ba2+. Group V Common cation, which do not react with reagents of the previous groups, form the last group of cations. They are: magnesium Mg2+, sodium Na+, potassium K+ and ammonium NH4+. 4 III. Exercise 1: Separation of I Group cations (Ag+, Pb2+, Hg22+) 1. Introduction Group I cations (Ag+, Hg22+, Pb2+) form insoluble chlorides. Upon the addition of hydrochloric acid Ag+, Pb2+, Hg22+ ions will precipitate as AgCl, PbCl2 and Hg2Cl2 AgNO3 + HCl AgCl + HNO3 Pb(NO3)2 + 2 HCl PbCl2 + 2 HNO3 Hg2(NO3)2 + 2 HCl Hg2Cl2 + 2 HNO3 (reaction 1) (reaction 2) (reaction 3) The solubility of PbCl2 increases approximately threefold as the temperature of the solution increases from 20°C to 100°C. Thus, PbCl2 will dissolve in hot water while AgCl and Hg2Cl2 remains insoluble. The presence of Pb2+ ions in obtained solution can be proved with KI, K2CrO4 and H2SO4 solutions. Their addition yield a golden yellow precipitates of PbI2 and PbCrO4 (reactions 4 and 5), and white precipitate of PbSO4 (reaction 6). Pb2+ + 2 KI PbI2 + 2 K+ Pb2+ + K2CrO4 PbCrO4 + 2 K+ Pb2+ + H2SO4 PbSO4 + 2 H+ (reaction 4) (reaction 5) (reaction 6) The precipitate still may contain Hg2Cl2 and AgCl. Of those two compounds, only the silver chloride is soluble in aqueous ammonia due to the formation a colorless solution of Ag(NH3)2Cl (reaction 7), whereas mercury(I) chloride turns into Hg metal and HgNH2Cl visible as grayish-black precipitate which is insoluble in ammonia solution (reaction 8) AgCl + 2 NH3 Ag(NH3)2Cl Hg2Cl2 + 2 NH3 Hg + HgNH2Cl + NH4Cl (reaction 7) (reaction 8) The formation of white precipitate of AgCl in reaction of Ag(NH3)2Cl with diluted HNO3 proves the presence of silver (reaction 9). The additional confirmation of silver ions is yellowish precipitate of silver iodide AgI obtained in the reaction with potassium iodide KI (reaction 10). Ag(NH3)2Cl + 2 HNO3 AgCl + 2 NH4NO3 Ag(NH3)2Cl + KI AgI + 2 NH3 + KCl (reaction 9) (reaction 10) 2. The identification of the sample composition To identify cations present in the given sample you can follow the Scheme 1 presented below with the use of flow chart. 5 Scheme 1. Analysis of the Group I. Procedure 1. Take 5 ml of a sample destined for identification and add 10 ml of 2M HCl. You should obtain white precipitate (Precipitate A). 2. Separate the precipitate by the filtration and wash it twice with cold distilled water and discard the washing. 3. Put clean test tube under the funnel with the precipitate. Then wash the precipitate with 10 ml of hot distilled water and collect the supernatant (Filtrate B). It is very important to remove all PbCl2 from the precipitate to follow further analysis of remaining AgCl and Hg2Cl2 which may be present in the Precipitate B. 6 4. Now you can confirm the presence of Pb2+ ions. In this purpose divide the solution of Filtrate B into three part and add KI, K2CrO4 and H2SO4 to each test tube respectively. Addition of those chemicals successively will yield a golden yellow precipitates of PbI2 (reaction 4) and PbCrO4 (reaction 5), and white precipitate of PbSO4 (reaction 6). 5. After confirming the presence of Pb2+ ions in the solution wash the Precipitate B with additional portion of hot water until the washing give no precipitate with K2CrO4 solution. White Precipitate B may still contain Hg2Cl2 and AgCl. Of those two compounds, only the silver chloride is soluble in aqueous ammonia due to the formation a colorless solution of Ag(NH3)2Cl (Filtrate C, reaction 7). Mercury(I) chloride reacts with ammonia solution to form grayish-black precipitate of Hg metal and HgNH2Cl which are insoluble in ammonia solution and remain on the filter (Precipitate C, reaction 8). 6. Pour ammonia solution onto the filter with Precipitate B and collect colorless solution in clean test tube (Filtrate C). Observe also the filter, because the precipitate may dissolve completely or turn black. If the color will change into black it means that Hg22+ is present in the initial sample. This is the final confirmation of the Hg22+ presence. The lack of precipitate indicate the absence of Hg2Cl2 and the lack of Hg22+ cations in the initial sample. Therefore, the last step is to follow the test for Ag + cations which is given below. 7. Divide the Filtrate C into two parts. To the first one add dilute 2M nitric acid HNO3 (reaction 9). The formation of white precipitate of AgCl prove the presence of silver. To the second test tube add the solution of potassium iodide KI. Yellowish precipitate of silver iodide AgI is the additional confirmation of the presence of silver ions in the sample (reaction 10). As you perform the experiment, collect discard all in the appropriate waste containers. DO NOT POUR ANY OF THE SOLUTIONS DOWN THE DRAIN. Results: Prepare your results for Group I on the report sheet provided. Be sure to include your all positive cations present in your initial sample. Use correct formulas for reagents and products. 7 IV. Exercise 2: Separation the II Group cations (Hg2+, Bi3+, Cu2+, Cd2+) 1. Introduction The Group II cations can be separated through selective precipitation of a number of sulfides. HgS, Bi2S3, CuS and CdS precipitate from reaction with H2S in 0.3M HCl (reactions 14). Hg2+ + H2S HgS(black) + 2 H+ 2 Bi3+ + 3 H2S Bi2S3(brown) + 6 H+ Cu2+ + H2S CuS(black) + 2 H+ Cd2+ + H2S CdS(yellow) + 2 H+ (reaction 1) (reaction 2) (reaction 3) (reaction 4) HgS, Bi2S3, CuS and CdS can be separated by differential solubility. HgS is insoluble in nitric acid while the Bi2S3, CuS and CdS are soluble. Bi2S3 + 8 HNO3 2 Bi(NO3)3 + 2 NO + 3 S + 4 H2O 3 CuS + 8 HNO3 3 Cu(NO3)2 + 2 NO + 3 S + 4 H2O 3 CdS + 8 HNO3 3 Cd(NO3)2 + 2 NO + 3 S + 4 H2O (reaction 5) (reaction 6) (reaction 7) Therefore, when the sulfide precipitate is treated with HNO3, HgS and S should remain as a precipitate while the rest of the cations should remain in solution. Although HgS does not dissolve in HNO3, it will dissolve in aqua regia (concentrated solutions of HCl and HNO3 acids mixed together): 3 HgS + 2 HNO3 + 6 HCl 3 HgCl2 + 2 NO + 3 S + 4 H2O (reaction 8) The resultant HgCl2 can be used to confirm the presence of Hg2+ in the reaction with tin(II) chloride. The reaction equation is as follows: 2 HgCl2 + SnCl2 Hg2Cl2 + SnCl4 Hg2Cl2 + SnCl2 2 Hg + SnCl4 (reaction 9) (reaction 10) Next cation which can be separated from the residual is Bi 3+. This cation forms white precipitate of Bi(OH)3 with NH3H2O. Cd2+ and Cu2+ also precipitate as white Cd(OH)2 and blue Cu(OH)2: Bi(NO3)3 + 3 NH3H2O Bi(OH)3 + 3 NH4NO3 2Cu(NO3)2 + 2 NH3H2O Cu2(OH)2(NO3)2 + 2 NH4NO3 Cd(NO3)2 + 2 NH3H2O Cd(OH)2 + 2 NH4NO3 (reaction 11) (reaction 12) (reaction 13) but they dissolve in an excess of NH3 solution, whereas Bi(OH)3 does not: Cu2(OH)2(NO3)2 + 4 NH3H2O Cu(NH3)4(NO3)2 + 6 H2O Cd(OH)2 + 4 NH3H2O Cd(NH3)4(OH)2 + 4H2O (reaction 14) (reaction 15) 8 Reaction of Bi(OH)3 with sodium stannite Na2SnO2 gives rise to black elemental bismuth which proves the presence of Bi3+ ions: 2 Bi(OH)3 + 3 Na2SnO2 2 Bi + 3 Na2SnO3 + 3 H2O (reaction 16) The solution that contain Cu(NH3)4(NO3)2 and Cd(NH3)4(OH)2 has deep blue color. To identify Cd2+ ions Cu2+ are reduced to colorless Cu+ with the use of KCN as a reducing agent. 2 Cu(NH3)4(NO3)2 + 10KCN + H2O 2K3[Cu(CN)4] + 4KNO3 + NH4CN + NH4CNO + 6NH3 (reaction 17) Cd(NH3)4(OH)2 + 4 KCN K2[Cd(CN)4] + 4 NH3 + 2 KOH (reaction 18) Reaction of Cd2+ with H2Saq in the resultant solution gives rise to the yellow sulfide precipitate of CdS: Cd(NH3)4(OH)2 + H2Saq CdS + 4NH3 + 2H2O (reaction 19) 2. The identification of the sample composition There is one test for Hg2+ which you can accomplish from the original sample. In this purpose take clean test tube with 3 ml of the initial sample and put tin copper plate into it. Wait for 2 minutes, then remove the plate, wipe it off and check whether it is covered with “argentic” layer. If yes, it means Hg2+ is present in the initial sample. Nevertheless to identify all cations in the given sample you should follow the Scheme 2 presented below with the use of flow chart. Procedure: Note: Students will use water saturated with H2S. The resultant solution is further written as H2Saq. 1. Pour 10 ml of your sample into a beaker and add 5 ml of 2M HCl and 120 ml of H2Saq. The precipitate may contain Group II sulfide precipitate: HgS, Bi2S3, CuS and CdS (Precipitate A reactions 1-4). 2. Filter the mixture in purpose of collecting both Precipitate A and the supernatant. Add again some H2Saq to the supernatant and check whether all sulfides precipitated. If you do not observe any precipitate after adding H2Saq you can discard this supernatant and continue the experiments with Precipitate A only. 9 3. In purpose of removing any residual Cl– from the resultant precipitate, wash Precipitate A with two portion of distilled water and discard the supernatant. Scheme 2. Analysis of the Group II. 4. Transfer the sulfide Precipitate A to a beaker and add 10 ml of 6M HNO3. Heat the mixture gently to almost boiling to ensure the sulfides, except HgS, have been dissolved by the HNO3 (reactions 6-9). 10 5. If HgS is present, the black precipitate should remain in the solution. Filter it and collect both the precipitate which may contain HgS (Precipitate B) and the supernatant with the remaining cations (Filtrate B). If you see some any pale or yellow precipitate it means that this is sulfur and Hg2+ is not present in the initial sample. Filter it anyway, discard the precipitate and collect the supernatant (Filtrate B) which may contain Bi3+ and Cu2+ and Cd2+ as nitrates. If Precipitate B is black from HgS save the filter for further analysis and identify the composition of Filtrate B. 6. Filtrate B may contain Bi3+, Cu2+ and Cd2+. The blue color of Filtrate B indicate the presence of Cu2+ ions, however to verify it, first Bi3+ ions have to be separated. 7. In this purpose add some concentrated NH3 solution to Filtrate B, stir the solution and check the pH with litmus paper until it turns blue color (reactions 11-15). Observe the resultant mixture whether some white precipitate of Bi(OH)3 is present. 8. Now filtrate it and collect both Bi(OH)3 as Precipitate C on a filter and Filtrate C in clean test tube. 9. Precipitate C contains Bi(OH)3. To verify it prepare the mixture of one portion of SnCl2 solution and one portion of 4M NaOH solution in purpose of obtaining colorless solution of Na2SnO2. Pour the resultant solution onto Precipitate C with Bi(OH)3. The appearance of a black precipitate on the filter proves the presence of bismuth (reaction 16). 10. If Filtrate C is colorless it means the copper is absent in the solution. If the color of Filtrate C is deep blue it means the copper is present in the solution and you have to reduce it to colorless Cu+ to identified Cd2+ ions (reactions 17-18). In this purpose add small amount of KCN solution until color disappears. Then treat the solution with H 2Saq. A yellow precipitate of CdS proves the presence of cadmium (reaction 19) 11. Now return to Precipitate B. Transfer it into a beaker and add few ml of concentrated HCl and a few drops of concentrated HNO3 and boil it (reaction 8). Evaporate the obtained solution, dilute it with distilled water and filter it. 12. Now add some SnCl2 to the supernatant which contains HgCl2. If Hg2+ is present you will obtain grey precipitate of Hg (reactions 9-10). As you perform the experiment, collect discard all in the appropriate waste containers. DO NOT POUR ANY OF THE SOLUTIONS DOWN THE DRAIN. Results: Prepare your results for II Group on the report sheet provided. Be sure to include all positive cations present in your sample. Use correct formulas for reagents and products. 11 V. Exercise 3: Separation of III Group cations (Co2+, Ni2+, Fe3+, Mn2+, Cr3+, Al3+, Zn2+) 1. Introduction Based upon previous laboratory experience, you may already have known that some ions can be recognized by the color of their solution: Ni2+ is pale green, Co2+ is pink, Fe3+ is usually yellow, Cr3+ is deep blue and Mn2+ is either colorless or very pale pink, depending upon the concentration. The solutions of remaining ions Zn2+ and Al3+ are colorless. The cations of Group III precipitate as CoS (black), NiS (black), Fe2S3(black), ZnS (white) and MnS (pale pink) sulfides and Cr(OH)3 (blue-green) and Al(OH)3 (white) hydroxide after addition of NH4Cl, NH3 and H2S. Co(NO3)2 + 2 (NH4)2S CoS + 2 NH4NO3 Ni(NO3)2 + 2 (NH4)2S NiS + 2 NH4NO3 Fe(NO3)3 + 3 (NH4)2S Fe2S3 + 3 NH4NO3 Zn(NO3)2 + 2 (NH4)2S ZnS + 2 NH4NO3 Mn(NO3)2 + 2 (NH4)2S MnS + NH4NO3 2 Cr(NO3)3 + 3 (NH4)2S + 6 H2O 2 Cr(OH)3 + 6 NH4NO3 + 3 H2S 2 Al(NO3)3 + 3 (NH4)2S + 6 H2O 2 Al(OH)3 + 6 NH4NO3 + 3 H2S (reaction 1) (reaction 2) (reaction 3) (reaction 4) (reaction 5) (reaction 6) (reaction 7) Nevertheless most of the tests for these ions involve the formation of a colored precipitate or complex ion (as is the case with Co2+, Ni2+ and Fe3+). Some of these tests are listed below: Ni2+ forms strawberry-red precipitate of complex with dimethylglyoxime (DMG) in basic solution Ni(NO3)2 + 2 HDMG Ni(DMG)2 + 2 HNO3 (reaction 8) Fe3+ reacts with KSCN forming dark-red solution of K3[Fe(SCN)6] Fe(NO3)3 + 6 KSCN K3[Fe(SCN)6] + 3 KNO3 (reaction 9) Co2+ in reaction with NH4SCN forms blue complex of (NH4)2[Co(SCN)4] which is soluble in amyl alcohol Co(NO3)2 + 4 NH4SCN (NH4)2[Co(SCN)4] + 2 NH4NO3 (reaction 10) Mn2+ oxidize the purple permanganate ion, MnO4– in the reaction with sodium bismuthate NaBiO3 in acidic solution. The appearance of a purple supernatant confirms the presence of Mn2+. 2 Mn(NO3)2 + 5 NaBiO3 + 16 HNO3 2 HMnO4 + 5 Bi(NO3)3 + 5 NaNO3 + 7 H2O (reaction 11) Al3+: The test for aluminum ion involves the adsorption of the red dye aluminon (aurin tricarboxylic acid) by aluminum hydroxide as the Al(OH)3 precipitates. 12 Cr3+: The test for chromium(III) ion involves its reduction by hydrogen peroxide in an excess of NaOH. The appearance of yellow solution confirms the presence of Cr3+ as CrO422 Na[Cr(OH)4] + 2 NaOH + 3 H2O2 2 Na2CrO4 + 8 H2O (reaction 12) Nevertheless to identify Zn2+, Al3+ and Cr3+ ions the remaining cations of Group III must be removed from the solution. In this purpose NaOH is used as a first. The hydroxides of all seven cations are at first precipitated: Co(NO3)2 + 2 NaOH Co(OH)2 + 2 NaNO3 Ni(NO3)2 + 2 NaOH Ni(OH)2 + 2 NaNO3 Fe(NO3)3 + 3 NaOH Fe(OH)3 + 3 NaNO3 Mn(NO3)2 + 2 NaOH Mn(OH)2 + 2 NaNO3 Zn(NO3)2 + 2 NaOH Zn(OH)2 + 2 NaNO3 Al(NO3)3 + 3 NaOH Al(OH)3 + 3 NaNO3 Cr(NO3)3 + 3 NaOH Cr(OH)3 + 3 NaNO3 (reaction 13) (reaction 14) (reaction 15) (reaction 16) (reaction 17) (reaction 18) (reaction 19) The hydroxides of zinc, aluminum and chromium are amphoteric and dissolve in an excess of NaOH to form complex ions: Zn(OH)2 + 2 NaOH Na2[Zn(OH)4] Al(OH)3 + NaOH Na[Al(OH)4] Cr(OH)3 + NaOH Na[Cr(OH)4] (reaction 20) (reaction 21) (reaction 22) Next hydrogen peroxide is used to oxidize Cr(OH)4-, Mn(OH)2 and Co(OH)2 Mn(OH)2 + H2O2 MnO(OH)2 + H2O 2 Co(OH)2 + H2O2 2 Co(OH)3 2 Na[Cr(OH)4] + H2O2 2 Na2CrO4 + 8 H2O (reaction 23) (reaction 24) (reaction 25) After separation of the precipitate by filtration CrO42- ions can be tested with the use of BaCl2. The appearance of yellow precipitate of BaCrO4 proves the presence of Cr3+ ions in the initial sample. Na2CrO4 + BaCl2 BaCrO4 + 2 NaCl (reaction 26) The same solution is kept for testing for Zn2+ and Al3+. The identification of aluminum is described above, whereas Zn2+ can be verified by the precipitation of white ZnS in reaction with H2Saq: Na2[Zn(OH)4] + H2S ZnS + 2 NaOH + 2 H2O (reaction 27) 13 2. The identification of the sample composition To identify Ni2+, Co2+, Fe3+ and Mn2+ cations present in the given sample you can follow separate tests for each of them and they are described below. Test for nickel, Ni2+ Take 2 ml of the initial sample and add some drops of 2M NH3 and do not shake the mixture. Next add few drops of dimethylglyoxime (HDMG) solution. The appearance of a strawberry red precipitate of Ni(DMG)2 indicates the presence of Ni2+ (reaction 8). Test for iron(III) Fe3+ and cobalt Co2+. Take 2 ml of the initial sample and add some drops of KSCN. The appearance of a blood-red color of the solution confirms the presence of iron(III) (reaction 9). Now add some drops of NaF until the solution is colorless. If you do not notice this color you do not have to add NaF. Next add one spoon of solid ammonium thiocyanate NH4SCN and approximately 2 ml of amyl alcohol. Shake the contents of the test tube vigorously and wait a few second until the the solution divide into layers and observe the color of the upper layer (organic). If the alcohol layer turns blue due to the [Co(SCN)4]2- complex ion formation, cobalt cations are present in the initial sample (reaction 10). If not, Co2+ ions are not present. Test for manganese, Mn2+ Take 1 ml of the initial sample and add 5 ml of 2M HNO3 solution and a few grains of sodium bismuthate NaBiO3. Allow to stand for a while. The appearance of a purple color of the solution confirms the presence of Mn2+ (reaction 11). To identify Zn2+, Al3+ and Cr3+ ions you should follow the Scheme 3 presented below with the use of flow chart. Procedure: 1. Pour 5 ml of your sample containing Group III cations to a beaker and add some grains of solid NaOH, 5 ml of 4M NaOH solution and a few drops (maximum one spoon) of hydrogen peroxide H2O2. Next boil the resultant mixture for ca. 15 min. 2. Filtrate the mixture. Discard Precipitate A and collect Filtrate A which may contain CrO42as yellow and colorless Al(OH)4- and Zn(OH)42-. If Filtrate A is colorless it means that Cr3+ are absent in the initial sample and you can skip step 4 of this procedure. 3. Cool the solution of Filtrate A and add concentrated CH3COOH until the solution is just acidic to orange litmus paper. Be sure to monitor this step carefully in order to get the pH into the right range. 4. To the solution obtained in step 3, potentially containing CrO42-, Al(OH)4- and Zn(OH)42add BaCl2 solution. Yellow Precipitate B of BaCrO4 is the final confirmation of the presence of Cr3+ in the initial sample. Filter it and collect the supernatant of Filtrate B. 14 5. Filtrate B should be tested for the presence of Al3+ and Zn2+. Divide this solution into two parts. To the first part add some of H2Saq solution. Any Zn2+ present will appear immediately as white or greyish precipitate. Scheme 3. Analysis of the Group III. 6. To the second part of Filtrate B add 1 ml of aluminon dye, and boil the solution in a test tube. Be careful during boiling process and shake test tube vigorously. Then add 2M NH3 until the solution is basic. You can also add some (NH4)2CO3. Any Al3+ present will form a gelatinous precipitate of Al(OH)3 that absorbs the red dye to give red precipitate which confirms the presence of Al3+. As you perform the experiment, collect discard all in the appropriate waste containers. DO NOT POUR ANY OF THE SOLUTIONS DOWN THE DRAIN. Results: Prepare your results for Group III on the report sheet provided. Be sure to include your unknown number and the positive cations present in your unknown. Use correct formulas for reagents and products. 15 VI. Exercise 4: Separation of IV-V Group cations (Ca2+, Ba2+, Mg2+, K+, Na+, NH4+) 1. Introduction In a mixture of Groups IV and V cations, Group IV cations form insoluble carbonates on addition of ammonium carbonate (NH4)2CO3 in the presence of NH3 and NH4Cl solutions. Since the remaining Group V ions (Mg2+, Na+, K+) form soluble salts with CO32-, they remain in solution. This makes them easy to separate from the solid IV Group carbonates. Ba(NO3)2 + (NH4)2CO3 BaCO3 + 2 NH4NO3 Ca(NO3)2 + (NH4)2CO3 CaCO3 + 2 NH4NO3 (reaction 1) (reaction 2) The elements of Group IV belong to the same periodic table group. As a result they have very similar physical properties: carbonates of Ca2+ and Ba2+ are soluble in acetic acid with the simultaneous evaporation of carbon dioxide: BaCO3 + 2 CH3COOH (CH3COO)2Ba + H2O + CO2 CaCO3 + 2 CH3COOH (CH3COO)2Ca + H2O + CO2 (reaction 3) (reaction 4) Ba2+ ions precipitate as yellow BaCrO4 in the reaction with K2CrO4 which is not soluble in acetic acid CH3COOH: (CH3COO)2Ba + K2CrO4 BaCrO4 + 2 CH3COOK (reaction 5) whereas Ca2+ results in white precipitate in reaction with ammonium oxalate (NH 4)2C2O4: (CH3COO)2Ca + (NH4)2C2O4 CaC2O4 + 2 CH3COONH4 (reaction 6) Flame tests is also used for the identification of Ca2+ and Ba2+: Ca2+ gives a brick red flame, and Ba2+ gives a yellow-green flame. Group V cations do not react with reagents of the previous groups. However Na+ and K+ ions are easily distinguishable by the distinctive color they give to a flame when their solutions are burned: Na+ gives a bright yellow flame, and K+ gives a violet flame. The presence of K+ ions can be also verified in a reaction with perchloric acid HClO4 which results in white precipitate of KClO4: KNO3 + HClO4 KClO4 + HNO3 (reaction 7) Magnesium cations do not give any color flame, however they react with Na2HPO4 and NaOH giving white precipitates of MgNH4PO4 (reaction 8) and Mg(OH)2 (reaction 9). But first, Ca2+ and Ba2+ have to be separated from a mixture. Additionally, an excess of NH4+ ions preserve Mg2+ before precipitation as Mg(OH)2 with carbonates of Group IV cations. Mg(NO3)2 + NH3 + Na2HPO4 + 5 H2O MgNH4PO4 + 2 NaNO3 Mg(NO3)2 + 2 NaOH Mg(OH)2 + 2 NaNO3 (reaction 8) (reaction 9) 16 The NH4+ ion is also a member of Group V cations, but is also introduced as a contaminant in the separation of Ca2+ and Ba2+ as carbonates in a reaction with (NH4)2CO3. Therefore it can be tested using the original initial sample to which no ammonia or ammonium salts have been added as reagents. They easily evaporate from the solution in a reaction with strong base: NH4NO3 + NaOH NH3 + NaNO3 (reaction 10) 2. The identification of the sample composition To identify all cations present in the given sample use separate tests for NH 4+ and K+ described below. NH4+ can be verify in the reaction with strong base. In this purpose take the small glass object, put some grains of solid NaOH on it and pour your analyzed sample. Mix it with glass rod and smell it. The evolution of ammonia can be detected by its odour (Caution!). K+: take 2 ml of the initial sample to the test tube and add 1 ml of HClO4 acid. If K+ is present you should observe the formation of white precipitate of KClO4. Remember to carry this test out at the beginning of your experiments before the precipitation of Group IV cations. To verify the presence of the Ca2+, Ba2+ and Mg2+ follow the Scheme 4 with procedure described below. Procedure: 1. Pour 5 ml of the examined sample into a beaker and add some of 2M NH3 and NH4Cl solutions and then 10 ml of (NH4)2CO3 solution. You will observe white precipitate which may contains CaCO3 and BaCO3 (Precipitate A). 2. Boil it for ca. 10 min. 3. Filter it and collect both Precipitate A and Filtrate A. You will analyze Filtrate A later in order to check the presence of Mg2+ ions in it. Now you are going to work with Precipitate A. 4. In this purpose put clean test tube under the funnel and wash the filter with 4 ml of 2M CH3COOH. Collect the supernatant which may contain Ca2+ and Ba2+ (Filtrate B). 5. Add 5 ml of K2CrO4 to Filtrate B. A yellow precipitate of BaCrO4 prove the presence of Ba2+ ions in the examined sample (Precipitate C). The lack of a precipitate automatically prove their absence. 6. Filter the mixture in the event that precipitate occurs and collect the supernatant which may contain Ca2+ ions (Filtrate C). 17 Scheme 4. Analysis of the Group V 7. Take Filtrate C and add some of 2M (NH4)2C2O4. Any Ca2+ present will appear immediately as white precipitate of CaC2O4. 8. Now return to Filtrate A saved in step 3 of the procedure. Divide this solution into two parts. To the first one add some of diluted NaOH. Mg(OH)2 will occur as colorless gelatinous precipitate. To the second part of Filtrate A add some of 2M NH3 and Na2HPO4 solution. White precipitate of MgNH4PO4 proves the presence of Mg2+ ions in the examined sample. As you perform the experiment, collect all waste solutions in a waste beaker and discard them in the appropriate waste container. DO NOT POUR ANY OF THE SOLUTIONS DOWN THE DRAIN. Results: Prepare your results for Group IV and V on the report sheet provided. Be sure to include all cations present in your unknown. Use correct formulas for reagents and products. 18