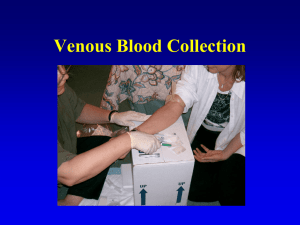
A Guide to Safe Phlebotomy Participant
advertisement

Ministry of Health Safe Phlebotomy Training for Health Care Workers in Kenya Participant’s Manual 2013 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Copyright © 2013, Ministry of Health, Government of Kenya The Safe Phlebotomy Training Curriculum is a comprehensive approach to the training of health care workers. The other components in this package are: • Trainers’ Manual • Curriculum Outline Enquiries regarding these Safe Phlebotomy Documents should be addressed to: Head National AIDS and STI Control Programme (NASCOP) Ministry of Health P.O. Box 19361 - 00202 Nairobi, Kenya Telephone: +254 20 2729502/2729549 Fax: +254 20 271 0518 or 272 9502 Email: head@nascop.or.ke Website: www.nascop.or.ke Recommended Citation National AIDS & STDs STI Control Programme (NASCOP), Ministry of Health Kenya. 2013. Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual. This publication was supported by Cooperative Agreement Number 1U2GPS001862 from the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention. 2 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 CONTENTS Acronyms and Abbreviations.............................................................4 Acknowledgements..........................................................................5 Introduction....................................................................................6 Background....................................................................................7 Understanding the Training Manuals..................................................8 Course Syllabus...............................................................................8 Broad Objectives.............................................................................9 Course Content...............................................................................9 Training Methodology.......................................................................10 Target Group...................................................................................10 Duration of Training.........................................................................10 Certification....................................................................................11 Training Schedule............................................................................13 Module 1........................................................................................13 Module 2.......................................................................................18 Module 3.......................................................................................30 Module 4.......................................................................................48 Module 5.......................................................................................59 Module 6.......................................................................................70 Module 7.......................................................................................83 Module 8.......................................................................................92 Module 9.......................................................................................97 Additional Resources.......................................................................102 3 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 ACRONYMS AND ABBREVIATIONS AIDS BD CDC DBS DH EDTA FEFO FIFO HAI HBV HCV HCW HIV IDSR IV KAIS KEMRI KMTC LCD MOH MOU MSH NASCOP NEMA NSI OGAC PEP PEPFAR PGH PPE PPP SOPs TOT UON WHO Acquired Immune Deficiency Syndrome Becton Dickinson Centres for Disease Prevention and Control Dried Blood Spot District Hospital Ethylene Diamine Tetra Acetic Acid First Expiry First Out First In First Out Hospital Acquired Infections Hepatitis B Virus Hepatitis C Virus Health Care Workers Human Immunodeficiency Virus Integrated Disease Surveillance And Response Intravenous Kenya AIDS Indicator Survey Kenya Medical Research Institute Kenya Medical Training College Liquid Crystal Display Ministry of Health Memorandum of Understanding Management Sciences for Health National AIDS & STDs Control Program National Environmental Management Authority Needle Stick Injuries Office of Global AIDs Coordinator Post Exposure Prophylaxis President’s Emergency Plan for AIDS Relief Provincial General Hospital Personal Protective Equipment Public-Private Partnership Standard Operating Procedures Training of Trainers University of Nairobi World Health Organization 4 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 ACKNOWLEDGEMENTS The following institutions are acknowledged for their contribution in the development of the Safe Phlebotomy Training Curriculum: • • • • • National AIDS & STI Control Programme Becton, Dickinson Company US Office of the Global AIDS Coordinator (OGAC-PEPFAR) US Centers for Disease Control and Prevention Management Sciences for Health The following individuals dedicated their time and tirelessly contributed towards the development of this manual. Abdille Ali Beatrice Kipesha Beatrice Njoki Carren Ogutu Catherine Gichimu Clement Kalesingor Dr Kelvin Okoth Dr. Gabriel Mngola Dr. Patrick Mwangi Dr. Paul Njanwe Dr. Robbinson Nduati Fridah Sirima Jacob Okello Japheth Gituku Jayne Munyao Joseph Mwangi Martin Mudogo Nancy Bowen Paul Okumu Peter Kariuki Peter Mbugua Rachel Chege Stephen Maina Winnie Migwi Zacharia Abukutsa Garissa Provincial General Hospital Coast Provincial General Hospital Embu Provincial General Hospital Siaya District Hospital Management Sciences for Health Kapenguria District Hospital Migori District Hospital Coast Provincial General Hospital University of Nairobi Kitale District Hospital Thika District Hospital Busia District Hospital Management Sciences for Health National AIDS & STI Control Programme Kakamega Provincial General Hospital Kenya Medical Research Institute Butere District Hospital National AIDS & STI Control Programme Nyanza Provincial General Hospital Nakuru Provincial General Hospital Kenya Medical Training College Nyeri Provincial General Hospital Becton Dickinson Company Nakuru Provincial General Hospital Kakamega Provincial General Hospital 5 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 INTRODUCTION Phlebotomy practices pose a risk of infection to health care workers, patients, and the community. The risk of exposure is related to service delivery as well as handling of medical waste. As a result, the Ministries of Health through the National HIV/AIDS and STI Control Programme (NASCOP) has developed a programme to address safe phlebotomy practices. Phlebotomy is a complex procedure, requiring both knowledge and skills. The phlebotomist must be knowledgeable in anatomy and physiology, be trained in all aspects of blood collection, and possess sufficient skill to perform the procedure safely. The importance of obtaining a good blood specimen cannot be overemphasised. Blood specimens are used in diagnosis of medical conditions, and to measure the level of medications in the patient’s blood to determine the effectiveness of patient management. Therefore, the integrity of the blood specimen is vital. The majority of laboratory errors occur during the pre-analytical phase. Several factors impact specimen quality: patient preparation, specimen collection equipment, collection technique, safety concerns, specimen handling, specimen transport, specimen processing, and specimen storage. Maintaining specimen quality therefore requires institutions and health care workers to be committed to acquiring knowledge and skills in order to reduce the errors. Institutions should implement an occupational exposure control plan that specifically addresses health care worker safety. Technology has evolved over time to address these concerns by providing a variety of safe equipment, including safety-engineered blood collection devices. Manufacturers respond to market forces and continue to work with the health care community to innovate and develop even safer devices. It is the responsibility of the health care community to advocate for safer medical devices so that procedures such as blood collection are no longer considered high risk. 6 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 BACKGROUND The risk of hospital-acquired infection (HAI) is universal and pervades every health care facility and system around the world. There are an estimated 35 million health care workers in the world and an estimated 2 million needle stick injuries (NSIs) annually, potentially exposing those workers to hepatitis B (HBV), hepatitis C (HCV), HIV, and other infections. In fact, this estimate is probably low, because of the lack of surveillance systems and the under-reporting of injuries. Research reported by the Global Occupational Health Network has shown 40-75% under-reporting of NSIs.1 The World Health Organization (WHO) estimates that the global burden of disease from occupational exposure to HBV and HCV is approximately 40% for each, and 4.4% of the HIV infections among health care workers.2 By 2007, 3 million Kenyans were being tested for HIV annually. At the time, 1.4 million Kenyans were living with HIV, of whom 750,000 were receiving care and treatment, thereby requiring regular blood tests.3 Since then, there has been a programmatic scale-up of HIV and AIDS interventions. The KAIS 2012 preliminary report estimate that, slightly more than 100,000 children aged between 18months to 14 years and approximately1,200,000 adults aged between 15- 64 years are living with HIV of these, 58% of the PLWHA require ARVs and subsequently blood draws in their management. With expansion of HIV testing, care, and treatment, there has been a rapid increase in blood collection. NASCOP initiated the Safe Phlebotomy project under the Injection Safety programme in 2006, with the aim of reducing the occupational risks from blood-borne pathogens. This culminated in the signing of a memorandum of understanding between Becton, Dickinson (BD) and the Office of the Global AIDS Coordinator (OGAC-PEPFAR) in 2009 as a public-private partnership activity based on a common agenda of strengthening health worker and patient safety through appropriate blood-drawing practices and laboratory services in countries severely affected by the HIV and AIDS pandemic. 7 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Understanding the Training Manuals The National Safe Phlebotomy Curriculum for Health Care Workers in Kenya is comprised of a package of four key components. These are the Curriculum Outline, Trainer’s Manual, Participant’s Manual, and the nine teaching modules (presentation slides). Course Syllabus Participant’s Manual Description The profession of phlebotomy is taught through didactic, student laboratory, and clinical experiences. The participant will be trained to perform a variety of blood collection methods using proper techniques and equipment—including vacuum collection devices, syringes, capillary skin puncture, butterfly needles, and blood culture specimen collection in adults, children, and infants. Emphasis will be placed on infection prevention, including safety and post-exposure prophylaxis (PEP), proper patient identification, proper labelling of specimens, and specimen handling, with emphasis on specimen quality, a component of quality assurance. Participants will also learn how to perform proper and safe blood draws using manikins—a skills-based approach—before performing further blood draws on patients in their institutions to enhance the skills learned during training. A teach-back methodology will be used to assess the trainee’s capacity to deliver the course to their colleagues. This Participant’s Manual therefore acts as a road map to guide the trainee through the course. It offers information on the following aspects pertinent to effective course delivery: • • • • • Course syllabus Course schedule Pre- and post-test Health care worker survey End of course evaluation Course Goal The overall goal of the Safe Phlebotomy Training is to improve the safety of health care workers, patients, and the community, as well as to improve the quality of specimens and other blood-drawing procedures critical to patient management. 8 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Broad Objectives The objectives of the course are to: 1. 2. 3. 4. 5. 6. 7. 8. 9. Explain the importance of safety and quality as core concepts in phlebotomy Describe phlebotomy equipment and supplies Equip participants with the skills for successful specimen collection by venipuncture Provide participants with knowledge and skills on equipment and techniques used in special conditions Provide participants with knowledge on complications arising from venipuncture and skills for their management Equip participants with knowledge and skills in capillary blood collection Furnish participants with knowledge, skills, and attitudes on biosafety practices Equip participants with the knowledge to manage occupational exposure to blood and body fluids Equip participants with knowledge and skills in inventory management Course Content The course covers the following nine modules: • • • • • • • • • Module Module Module Module Module Module Module Module Module 1: 2: 3: 4: 5: 6: 7: 8: 9: Importance of Safe Phlebotomy in Patient Care Phlebotomy Equipment and Supplies Successful Specimen Collection by Venipuncture Special Techniques in Specimen Collection by Venipuncture Complications during Specimen Collection by Venipuncture Capillary Blood Collection Safety and Infection Control Occupational Exposure Inventory Management Training Methodology This course uses a skills-based learning approach. The approach presupposes that all participants will acquire the knowledge, skills, and attitudes to perform safe phlebotomy. To determine participants’ level of knowledge, skill, and attitude acquisition, a pre-course assessment is given before training and a post-course test after training. The approach further appreciates that people not only learn best in different ways but also vary in their abilities to absorb new information. A variety of training methods and techniques suitable for adult learners have therefore been carefully selected to address individual differences as well as provide an enjoyable way of learning. These include lectures, quizzes, group discussions, demonstrations, role plays, video/ slide shows, practicals, teach back, and evaluation (pre- and post-test and course evaluation). The course takes very practical and participatory approaches, moving away from the traditional teacher-student model in which the teacher is held to be the expert. The approach assumes that participants are: 9 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 • Bringing with them a wealth of experience • Willing to be actively involved in training activities in order to acquire new skills or sharpen those they already have and gain competency in safe phlebotomy • Interested in helping to improve phlebotomy practices in their health facilities and having a positive impact on patient management Target Group Participants should be students from medical institutions and health care workers involved in blood drawing. Duration of Training The duration is two days training (theory and practical) and one day of teach back and work plan development. The participants will be assigned topics for teach back in the second day by the trainers. Certification Upon successful completion of the course, participants will be certified by NASCOP and the collaborating institution. 10 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 TRAINING SCHEDULE Day 1 Time Title 8:00–8:30 Registration 8:30–9:00 Introductions and Climate Setting • Expectations • Group norms • Administrative issues 9:00–9:15 Agenda • Opening remarks 9:15–9:45 Healthcare Worker Survey 9:45–10:15 Pre-training Phlebotomy Quiz Tea/coffee break 10:45–11:45 Module 1: Importance of Safe Phlebotomy in Patient Care 11:45–13:00 Module 2: Phlebotomy Equipment and Supplies Lunch break 14:00–16:00 Module 3: Successful Specimen Collection by Venipuncture 16:00–16:45 Module 4: Special Techniques in Specimen Collection by Venipuncture 16:45–17:00 Memory Jogger and Close Each participant notes down what changes he/she would make in procedure after learning from the day. Facilitators’ review meeting 11 Facilitator Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Day 2 Time 8:00–8:15 8:15–9:00 Title Review of previous day and questions Module 5: Complications During Blood Collection by Venipuncture 9:00–10.00 Module 6: Capillary Blood Collection 10:00–10:15 Tea/coffee break 10:15–11:00 Module 7: Safety and Infection Control 11:00–11:45 Module 8: Post-exposure Prophylaxis 11:45–12:30 Module 9: Inventory Management 12:30–13:30 Lunch break 13:30–14:30 Hands-on with Training Arm • Hand washing demo • Venous blood draws • Special venous collection 14:30–15:30 Hands-on • Finger prick • Heel prick 15:30–17:00 Post-test Evaluation Closing remarks 17:00 Tea/coffee break 17:00–17:30 Facilitators’ review/planning meeting Facilitator Day 3 Coaching of participants in clinical setting Perform four successful venipunture and four capillary blood draws (two heel pricks and two finger pricks) 12 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 1 Importance of Safe Phlebotomy in Patient Care Aim To introduce the importance of safety and quality as core concept in phlebotomy Prerequisite Modules None Objectives At the end of this module you will be able to: 1. Describe the significance of safety in phlebotomy 2. Describe the importance of specimen quality 3. Describe the chain of infection and preventive measures Content Outline • Introduction; definition of phlebotomy and the importance of phlebotomy in patient care • Safety of patients, HCWs, and the community • Quality of specimen in patient care and clinical decisions • Mode of transmission, port of entry, port of exit, reservoir host, susceptible host, breaking the chain of infection Notes on Customisation 13 Module 1 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Introduction and Definition What is phlebotomy? The term phlebotomy is from the Greek phlebo, meaning related to veins, and tomy, meaning related to cutting. Phlebotomy is therefore opening a vein to collect blood: it is the practice of drawing blood. What is the importance of phlebotomy? Phlebotomy is important in diagnostic testing of diseases, therapeutic assessment, and monitoring of the patient’s condition. Safety In phlebotomy, the safety of the patient, HCWs, and the community is the overall goal. The sample collected should be a true representation of the patient’s condition. The need for safety in phlebotomy is supported by the following facts documented by WHO and the Kenya AIDS Indicator Survey (KAIS): There are an estimated 35 million HCWs in the world and an estimated 2 million needle stick injuries (NSIs) annually, potentially exposing those workers to HBV, HCV, HIV, and other infections. This estimate is probably low, because of the lack of surveillance systems and the under-reporting of injuries. Research has shown 40–75% under-reporting of NSIs. WHO estimates that the global burden of disease from occupational exposure to HBV and HBC is approximately 40% for each, and 4.4% for HIV infections among HCWs (Prüss-Ustün). In Kenya, over 1.4 million people are living with HIV, of whom 750,000 are receiving care and require regular blood tests. Three million Kenyans are tested for HIV status annually. Besides HIV, other infections can be contracted thorough NSIs, as shown in the chart below. 14 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Diseases That CanbeBe Contracted List of diseases that can contracted through Needle-stick injuries through NSIs Module 1 Viral Infections Bacterial Infections Fungal Infections Hepatitis B Brucella abortus Blastomyces dermatidis Hepatitis C Corynebacterium diptheriae Cryptococcus neoformans Hepatitis G Neisseria gonorrheae Sporotrichum schenkii Human immunodeficiency virus Leptospira Icterohaemorrhagiae Simian immunodeficiency virus Mycobacterium marinum Protozoal infections Herpes simiae Mycoplasma caviae Plasmodium falciparum Herpes simplex Orientia tsutsugamushi Toxoplasma gondii Herpes zoster Rickettsia rickettsii Ebola/Marburg Staphylococcus aureus Dengue Streptococcus pyogenes Creutzfeldt-Jakob disease Treponema pallidum Mycobacterium tuberculosis Modes of Transmission Chain of Infection The chain of infection can be broken by effective hand hygiene procedures, immunisation against HBV, proper decontamination of surfaces and instruments, proper disposal of sharps and infectious waste, use of gloves, gowns, mask respirators, and other personal protective equipment (PPE), and safer and proper use of equipment. Infectious Agent Susceptible Host Reservoir Chain of Infection Portal of Entry Portal of Exit Mode of Transmission 15 Module 1 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Specimen Quality A specimen is not just a tube but a true representation of the patient’s condition. Only when a quality sample is produced can the laboratory produce valid results, which will enable the clinician to make an informed judgement based on the best possible data. Large numbers of clinical decisions are based on laboratory tests results. Poor sample quality affects the reliability of the results, which, in turn impacts doctors’ ability to provide quality patient care. Laboratory errors therefore affect the patient, clinician, laboratory, and the entire hospital. Some research has indicated that laboratory errors occur more frequently than expected. One study (Plebani and Carraro 1997) showed that one error occurs for every 214 laboratory results. Laboratory errors may occur either in the pre-analytical, analytical, or post-analytical phase of sample analysis. Up to 68% of laboratory errors occur in the pre-analytical phase, where most of the blood draws are done; 13% in analytical; and 19% in the post-analytical phase of sample analysis. Factors that impact specimen quality include patient preparation as well as specimen collection equipment and technique, handling, transportation, processing, and storage. Therefore, observation of the factors to the right contributes to the delicate link between the clinician, patient, and the hospital. Improvement in sample quality leads to increased reliability of test results, reduced errors, and better patient care and safety. Steps in Patient Sample Testing 16 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 1 Sample Rejection A blood sample will not be accepted for analysis if it does not meet the standard of quality. Recommended rejection criteria include haemolysis, insufficient sample, wrong sample container, leaking sample, etc. A rejection stamp is a tool used to ensure smooth communication. Sample rejection should also be documented in the laboratory. Key Messages 1. Safety for patients, HCWs, and the community is the ultimate goal in sample collection. 2. Phlebotomy also plays a key role in the intervention of transmission of blood-borne diseases. 17 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 2 Phlebotomy Equipment and Supplies Aim To introduce you to phlebotomy equipment and supplies Prerequisite Modules Module 1: Importance of Safe Phlebotomy in Patient Care Objectives By the end of this module you will be able to: 1. Identify equipment and supplies used in venous blood collection 2. Describe the purpose and importance of equipment 3. Differentiate between open and closed systems of phlebotomy Content Outline • Equipment and supplies • Application of equipment: different colour-coded tubes, tourniquet, etc. • Open system; syringe and needle • Closed system; evacuated system. Notes on Customisation 18 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 2 Types of Equipment There are various types of equipment and supplies used in venous blood collection. These include the following: • • • • • • • • • Tourniquet Gloves Antiseptic and disinfectant Needle Syringe or needle holder Specimen container Gauze Tape or bandage (strapping) Sharps container Tourniquets This is a stretchable strip of material 35–45 cm (15–18 inches) in length, and may be single use or re-usable. There are different types of tourniquets: latex, vinyl, elastic bands, etc. • • • Latex is the most commonly used because it is easily available and cheap. Vinyl is useful where the HCW or patient is allergic to latex. Elastic bands with Velcro® or buckle closure may also be used. Tourniquets should be discarded after use or cleaned with alcohol at the end of the procedure on each patient. Tourniquet application time = maximum 1 minute. 19 Module 2 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Use and Application of Tourniquets • • • Tourniquets make the veins easier to locate and feel. They also slow down venous blood flow and enlarge the veins. The tourniquet should not restrict arterial blood flow into the limb. Wrap 7.5–10.0 cm (3–4 inches) above the intended venipuncture site and tie so that it is releasable with one hand.. Gloves Gloves are sterile or clean coverings for the hands, usually with a separate sheath for each finger and thumb. They are part of personal protective equipment (PPE) against contact with blood during phlebotomy. They provide a barrier between the user and the patient, and hence prevent the spread of infection. A new pair should be worn for each patient and for each procedure to avoid infection crossover. A good fit is essential for effective infection prevention. Washing or reuse of gloves can compromise integrity of the material to serve as a barrier without showing any visible changes. Types of Gloves Three types of gloves are commonly used: latex, nitrile, and vinyl. Latex: Powdered latex gloves have cornstarch added to them to help the user who is donning them (putting them on) to slide their hands in more easily and more quickly. Latex gloves are comfortable, fit well, provide good barrier protection, and have tactile sensitivity. They are the most commonly used of the three common types. Nitrile: Nitrile is made from a synthetic polymer in the form of a latex or emulsion and can be used or processed very much like natural rubber latex. They fit well and provide good protection. 20 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 2 Vinyl: Research has shown that vinyl gloves are not as strong as latex or nitrile gloves in terms of their puncture resistance. They fit loosely to the hands due to their limited ability to stretch. They are mostly used for non-medical purposes. Vinyl gloves are too loose fitting and do not provide an adequate barrier to viruses. Antiseptics and Disinfectants Antiseptics inhibit or prevent the growth of bacteria, are approved for use on the skin, and are recommended for cleaning the venipuncture site. The most commonly used antiseptic is 70% isopropyl alcohol. Disinfectants kill bacteria and inhibit some viruses. Disinfectants are used on surfaces and instruments, but are not recommended for use on the skin. They are used to clean up all blood spills. The commonly used disinfectant is 1:10 hypochlorite solution (bleach). Antiseptics versus Disinfectants Antiseptics • • • • • Inhibit growth of bacteria Are used on the skin Evaporate in open air Are recommended for venipuncture Example: 70% isopropyl alcohol Disinfectants • • • • • Kill bacteria and some viruses Are used to clean surfaces and instruments, but not skin Do not evaporate Are recommended for cleaning spills Example: 1:10 hypochlorite solution (bleach) Isopropyl alcohol as an antiseptic Isopropyl alcohol is used in an optimal concentration of 70% as an antiseptic. Directions for use It is stored in a closed container to prevent the evaporation of the active ingredient. The active ingredient will evaporate from an open container, leaving water behind and causing the antiseptic properties to diminish. This renders pre-soaked cotton balls ineffective. Bacteria from hands and the container can multiply and cause infection in the patient if alcohol from an open container is used to “clean” the intended puncture site, and in addition the “cleaned” site may not dry quickly. NB: Never pre-soak cotton balls. 21 Module 2 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Needles A needle is a hollow stainless-steel shaft with lumen (the interior dimension) and bevel (the slant at the end). It is sterile, disposable, and for single use. Needles sizes Needles vary in sizes according to gauge. Gauge (G) refers to the diameter: The larger the number, the smaller the diameter of the needle. Different colour codes are used for different gauges, as shown. Length varies from 0.5–1.15 inches. Selection of the needle is based on: • Size of the vein • Location of the vein • Volume of the blood to be collected 22 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 2 Syringes The syringe is an instrument used to inject fluids into the body or draw them from it. It is made of three components, namely: 1. Plunger: Used to pull the blood fluid into the barrel 2. Barrel: Used to hold the syringe in place and contains the blood fluid 3. Needle hub: Attaches the barrel to the needle Sizes of Syringes There are different sizes of syringes: they range from 2 ml–20 ml Selection of size depends on: • Patient (e.g. geriatric, paediatric) • Volume of blood needed • Strength of vacuum expected Advantages • • Blood flash can be seen on entering vein User controls the amount of blood drawn Disadvantages • • Specimen can clot Specimen must be transferred Never exert pressure on the plunger in transfer to vacuum tube. 23 Module 2 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Systems of Blood Collection There are two systems of blood collection: 1. 2. Open system: Blood is collected using a syringe and needle. Closed system: Blood is collected using the evacuated system. Open System Open system: syringe and needle blood collection The vacuum to draw blood from the vein through the needle and into the syringe is created as the plunger is withdrawn. The vacuum is controlled by the user, as shown below. Reused prevention syringes should not be used for blood collection. Closed System Closed system: evacuation blood collection system The vacuum in the tube allows blood to be drawn directly from the vein into the evacuated tube, hence, there is no need to transfer blood. The vacuum in the tube controls the amount of blood to be drawn. 24 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Closed System Components Multiple-Specimen Collection Needles The multiple-specimen collection needle has two ends: 1. Patient end • Longer needle • Determines the gauge • Longer bevel to pierce patient skin and enter the vein 2. Non-patient end • Shorter needle • 20 G to minimise haemolysis • Penetrates rubber top of collection tube • Enclosed by a flexible rubber sheath to prevent blood leak between specimens 25 Module 2 Module 2 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Other Equipment Needle Holder The needle holder is a clear plastic device with two ends: a narrow end and a wider end. The needle is attached to the holder at the narrow end while the collection tube is inserted to the wider end. The needle holder has wings or extensions which act as a lever to exert pressure when inserting or removing tubes while keeping the needle steady. Use needles and holders from the same manufacturer to ensure compatibility. Gauze Pads and Bandages Gauze pads are used to apply pressure to the site after needle removal and they should be clean. Adhesive bandages or tape are used to secure gauze and should not be applied directly to the site without gauze pads. Cotton is not recommended, as fibres can stick to the site and initiate bleeding when removed. Do not use alcohol swabs on the site, because they cause irritation. Sharps Disposal Containers and Safety Devices These are containers designed for the safe disposal of sharps waste. Needles and holders or needles and syringes should be disposed of as a unit immediately after use. Needles should not be recapped, bent, or cut, to avoid accidental pricks. The sharps container should have a biohazard label affixed to it. 26 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 2 There are different types of sharps disposal containers, as shown. Engineered Safety Devices/ Mechanisms These are devices which are incorporated into the needle for the safety of the user. The sheathing device is on the non-patient end of the needle and the needle retraction device is on the patient end. Evacuated TuAbes These are specimen containers used for venous blood draw and have a sterile interior. They are for single use, draw a predetermined volume of blood based on a measured vacuum, and have a predetermined quantity of additive which enables the correct blood/additive ratio. These are some of the evacuated tubes. 27 Module 2 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Evacuated Tube Attributes • • • • • • • Colour coded according to additive contained in the tube Additive will either promote or inhibit clotting Tube sizes vary from 2–10 ml Tube selected varies by: Tests to be performed Volume of blood to be collected Tubes have expiration dates; Do not use a tube after its expiry date because it loses its predetermined vacuum Serum Tube – Red Top The red top evacuated tube has the following: • • • • • Additive: silicone coat Mode of action: it has a silica clot activator to accelerate clotting Clotting time: 60 minutes Closure colour: red Principal application: serum extraction for routine procedures Purple/Lavender Top Tube The purple top evacuated tube has the following: • • • • • Additive: EDTA anticoagulant Mode of action: it removes calcium (chelates) from the blood Clotting time: 30 minutes Closure colour: purple/lavender Principal application: whole blood for haematology testing, HbA1C, red cell folate, haemoglobin electrophoresis, etc. 28 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 2 Gold Top – Serum Gel Tube The gold top evacuated tube has the following: • • • • • Additive: silica clot activator to accelerate clotting Mode of action: contains a “gel” material between the cellular components of the specimen and the serum/plasma Clotting time: 30 minutes Closure colour: gold Principal application: serum extraction for routine procedures Green Top The green top evacuated tube has the following: • • • • Additive: heparin Mode of action: it blocks the action of thrombin and thus prevents conversation of soluble fibrinogen to insoluble fibrin Closure colour: green Principal application: plasma for clinical chemistry testing Grey Top The grey top evacuated tube has the following: • • • • Additives: anticoagulant additives fluoride oxalate and fluoride EDTA Mode of action: fluoride acts as a glycolytic inhibitor, hence stabilising glucose concentration in the blood Closure colour: grey Principal application: glucose testing, also used for lactose testing Light-Blue Top The light-blue top evacuated tube has the following: • Additives: anticoagulant additives—tri-sodium citrate • Mode of action: acts by removing calcium from the blood (reversible action if calcium is replenished) • Closure colour: light blue • Principal application: plasma for coagulation testing Key message It is important to understand the purpose and function of every piece of equipment before using it! 29 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 3 Successful Specimen Collection by Venipuncture Aim To equip you with skills for successful specimen collection by venipuncture Prerequisite Modules Module 1: Importance of Safe Phlebotomy in Patient Care; Module 2: Phlebotomy Equipment and Supplies Objectives By the end of this module you will be able to: 1. 2. 3. 4. Outline the steps of patient identification Describe standard precautions during venous blood collection Identify recommended venipuncture sites Perform successful venous blood collection Content Outline • • • • Patient requisition, introduction, reassurance, identification; and handling of difficult and special situations Safety precautions: hand washing, gloves, gowns, masks, sharps containers Site selection: antecubital fossa Venous procedure: Steps involved in venous blood collection Notes on Customisation 30 Module 3 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 What Defines Successful Blood Collection? Successful venous blood collection ensures: • • • Patient safety HCW safety Quality of the specimen Steps in Blood Collection Blood collection involves several steps, including patient requisition, patient interaction, safety precaution, selecting equipment, positioning the patient, site selection, tourniquet application, site cleansings, blood withdrawal, sharps disposal, sample mixing and handling, and sample transportation. Steps in Venous Blood Collection Patient requisition Patient interaction Standard precautions Selacting equipment Site cleansing Tourniquet application Site selaction Positioning the patient Perform venipuncture Sharps disposal Sample handling/mixing Sample transport The above steps are important during venous blood draw; hence all steps should be taken seriously. They are discussed in detail below. 31 Module 3 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Step 1 Patient Requisition The clinician initiates the diagnostic process by requesting laboratory tests. Information contained on a requisition form includes: • • • • • • • • • • Patient’s name* Patient’s date of birth (DOB)* Medical record number* – this number is unique for each patient Date requested Residence Specimen destination Patient’s ward, bed, and room location Name or code of the physician making the request Type of tests requested Information about patient status (e.g. potential bleeding disorders or puncture sites to be avoided) Step 2 Patient Interaction Approach the patient calmly but confidently with a soothing and reassuring tone of voice. Introduce yourself to the patient. If a child or infant, ask parents to assist. If an inpatient, state where you are from and the purpose of your visit. Also look for special notices in the room regarding the patient. Patient reassurance Explain the procedure to be performed, providing reassurance to the patient. Gain the patient’s confidence by assuring the patient that the puncture may be slightly painful and will take a short time. Patient identification Identify the patient by asking him/her to state full names and date of birth. Verify with the information provided on the requisition form. Obtaining a specimen from the wrong patient can have serious, even fatal consequences, as in the case of specimens for typing and cross-match before transfusion. Where there is an identification discrepancy, the specimen must not be obtained until the discrepancy is resolved and the patient identification is verified. In the ward, ask patient name, verify name and medical record number on identification band, and match with the requisition form. Also verify patient with ward staff if an identification band is not available. In case of a young, mentally incompetent, or unconscious patient, you may ask the nurse, attendant, or relative to identify the patient. Also look for special notices in the room regarding the patient. 32 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 3 Patient preparation Be aware of specific dietary requirements for the requested test. Verify that special diet instructions have been followed. Fasting means refraining from eating for a preset time, as indicated by the clinician. Ask the patient the last time he/she took meals, and also inquire if the patient is hypersensitive to latex. Handling patient in special conditions In case the patient is asleep, gently wake by nudging the bed. Startling can cause a change in tests results. Anxiety and stress cause increased secretion of hormones (aldosterone, angiotensin, catecholamines, cortisol, prolactin, somatotropin, TSH, vasopressin) and increased concentrations of albumin, fibrinogen, glucose, insulin, and lactate in the patient. Step 3. Standard precautionAll specimens should be handled as if they were potentially infectious. Wash hands before and after patient care, wear protective gear such as gloves, dust coat, and mask, and avoid needle recapping. Step 3 Standard precautionAll specimens should be handled as if they were potentially infectious. Wash hands before and after patient care, wear protective gear such as gloves, dust coat, and mask, and avoid needle recapping. Hand Washing Hand washing helps to reduce the transmission of microbes acquired through contact. It also removes dirt and debris from the hands and protects the self and others. Hands should be washed in between patients, and after exposure to blood and other body fluids. 1. Wet hands with water 2. Apply enough soap to cover all hand surfaces. 33 Module 3 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 3. Rub hands palm to palm. 4. Right palm over left dorsum with interlaced fingers and vice versa. 5. Palm to palm with fingers interlaced. 6. Backs of fingers to opposing palms with fingers interlocked. 7. Rotational rubbing, with left thumb clasped in right palm and vice versa. 34 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 3 8. Rotational rubbing, backwards and forwards with clasped fingers of right hand in left palm and vice versa. 9. Rinse hands with water. 10. Dry thoroughly with a single-use towel. 11. Use towel to turn off faucet. 12. Your hands are safe. 35 Module 3 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Hand Sanitisers When soap and water are not available, hand sanitisers can complement infection control practices, but nothing can replace water and soap in achieving effective hand hygiene. How to Sanitise the Hands 1. Apply a palmful of the product in a cupped hand and cover all surfaces. 2. Rub hands palm to palm. 3. Right palm over left dorsum with interlaced fingers and vice versa. 4. Palm to palm with fingers interlaced. 5. Backs of fingers to opposing palms with fingers interlocked. 6. Rotational rubbing of left thumb clasped in right palm and vice versa. 7. Rotational rubbing, backwards and forwards with clasped fingers of right hand in left palm and vice versa. 8. Once dry, your hands are safe. 36 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 3 Wearing Gloves Since it may not be immediately evident that a specimen is infectious, all patient blood specimens should be treated as potentially infectious and handled according to standard precautions, which require the wearing of gloves during procedures involving blood/body fluids. Gloves provide a barrier to exposure to blood and fluids. A new pair of gloves should be worn for each procedure, and a good fit is essential. Step 4 Equipment Selection The appropriate equipment selected will be based on the patient, the procedure, and the test ordered. Assemble the eclipse needle and holder or needle and syringe. Remove needle sheath just before insertion into the vein. Step 5 Positioning the Patient The patient should be seated or lying down, but never standing or sitting on a high stool. For patients with a history of fainting, the appropriate positioning is lying down. The arm should be firmly supported and extended downwards, ensuring that it is kept straight from the shoulder to the wrist. The hand should be closed to make the veins prominent, but pumping of the fist must be avoided to prevent haemolysis. Positioning Paediatric Patients A child could have fear of unknown origin, and hence turn very aggressive. This could pose a risk to both self and the health care worker. Therefore, it is critical to restrict the movement of the child before the venipuncture procedure is initiated. Restrain the child by having them sit either beside the parent in a special chair or on the parent’s lap. The parent should wrap their arm around the child and over the arm that is not being used. The parent could also restrain the child by lying down, as shown above. 37 Module 3 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Step 6 Site Selection Blood is one of the most commonly analysed body fluids for diagnostic purposes. It is important to keep in mind that all veins are not suitable for venipuncture. The selected veins must meet some preset attributes that would help in making the specimen collection process least inconvenient to the patient whilst ensuring appropriate specimen quality. Attributes of Preferred Veins • • • • • Large enough to support good flow Easily visible Close to the skin surface Elastic – should not be hard to feel. Well anchored in surrounding tissue Vein Selection The major veins commonly used for venipuncture are located in the antecubital area. Veins of choice, in order of preference for venipuncture are: 1. 2. 3. Median cubital vein Cephalic vein Basilic vein Median cubital vein This is the first choice of vein because it is large, well anchored, least painful, and least likely to bruise. Cephalic vein This is the second choice of vein because it is large, not as well anchored, and may be more painful than the median cubital vein. Basilic vein This is the third choice because it is generally large and easy to palpate, but often not well anchored. It involves greatest risk because it lies near the brachial artery and median nerve, either of which could be accidentally punctured. • Piercing a nerve with a needle may cause ongoing pain and/or paralysis of the arm. • Scratching the brachial artery with a needle may cause hidden bleeding, leading to haematoma. 38 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 3 Palpating the Vein After vein selection, palpation of the vein is a necessary so as to identify whether the vein is suitable for puncture. This is achieved by pressing and releasing the vein several times. A thrombosed vein lacks resilience and should not be used. Rotating the arm slightly can help find a vein or differentiate a vein from other structures in the arm. Probing for a vein is never recommended. Inappropriate Sites for Venipuncture For safe blood collection and quality of blood sample, there are areas which are unsuitable for blood collection. These • • • • • • • include the following: Burns Edematous areas Scarred areas Haematomas Damaged veins (e.g thrombosed, non-elastic) Arm on side of mastectomy Sites downstream proximal from an IV line – these are sites which are closer to the heart and are above the IV line • Tattoo areas Step 7 Tourniquet Application A tourniquet makes the veins easier to locate and feel. It also slows down the venous flow, hence enlarging the veins. It should not restrict arterial blood flow into the limb. Wrap the tourniquet 7.5–10 cm (3–4 inches) above the intended puncture site. It should be tied to be releasable with one hand, and application time should not exceed one minute. 39 Module 3 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Tourniquet application procedure 1. Position the tourniquet 7.5–10 cm (3–4 inches) above the venipuncture site. 2. Cross the tourniquet over the patient arm. 3. Tuck a portion of one end under the opposite end to form a loop. 4. A properly applied tourniquet for easy release. Why the Maximum Time for Tourniquet Application is One Minute The recommended time of one minute ensures that the patient’s comfort is taken care of and there is slowed venous blood flow in and out of limb. Leaving the tourniquet in place for greater than one minute changes the concentration of blood components within the vein due to a biological process called haemo-concentration – small molecules (e.g. electrolytes and water) move from capillaries to interstitial space and the concentration of macromolecules (e.g. proteins, enzymes) left in the blood appears falsely elevated. Prolonged tourniquet application can also impact on other test values – e.g. platelet activation, which may lead to coagulation test errors. For example, a PTT (activated prothrombin time test) on patients receiving IV heparin because it leads to increase in the coagulation time. 40 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 3 Note: In case of unsuccessful blood draw, the tourniquet should be re-applied after two minutes. Step 8 Cleaning the Venipuncture Site This is a process which enables the phlebotomist to achieve the required cleanliness of the site. Start from the centre of the site and move outwards in ever-widening concentric circles. Failure to follow this procedure may reintroduce dirt and bacteria. Use sufficient pressure to remove surface dirt and debris. If the site remains dirty, repeat with a new swab. Allow to air-dry. Do not wipe, blow on, or fan, as these actions may reintroduce contaminant micro-organisms. Follow institutional procedure for blood cultures. Note: The site should not be touched after it is cleaned. Step 9 Needle Insertion Technique Angle of Insertion This technique involves two procedures; attaining the correct angle of needle insertion and correct needle insertion technique, as explained below. • • • √ Top: Bevel of needle fully inserted at the recommended 15–30-degree angle. × Middle: Angle too steep. Potential for needle to completely penetrate the vein, possibly resulting in formation of a haematoma. × Lower: Angle too shallow, bevel occluded by wall or partially in the vein/partially in tissue, possibly resulting in formation of a haematoma. 41 Module 3 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Insertion Technique • • • • • With needle cover removed, use dominant hand to grip holder/syringe (index and middle fingers below, thumb on top). Grasp patient’s arm (using non-dominant hand) with thumb on top, fingers wrapped to the back. Pull skin taut below the intended site with thumb, anchoring the vein to keep it from moving or rolling. Align with the vein (bevel up), in the same direction as venous flow. Use a smooth motion to quickly insert the needle. Stop needle advancement when slight decrease in resistance is felt, signalling entry into the vein. Areas of caution • • • • Needle contact with a nerve can cause pain and may induce involuntary reflex (pulling arm away from needle). Arteries, which can be detected by a pulse, should be avoided. To avoid accidental puncture, do not select a vein that overlies or is close to an artery. Excessive or blind probing can lead to permanent injury of the nerve or artery, which may result in permanent damage. Blood Collection There are two systems of blood collection: A. Evacuated / closed system B. Open system A. Evacuated / closed system In the closed system, the blood specimen is collected directly from the vein through the needle to the evacuated tube. After needle insertion, the procedure continues as explained below. The blood flows into the tube as shown below. 42 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 3 Tube Filling To ensure the continuous flow of blood into the tube, a downwards positioning of the tube should be maintained so that the blood and additive do not touch the non-patient end of the multi-sample needle. This will help prevent reflux of the anticoagulant. Allow the tube to fill until the vacuum is exhausted and the blood flow stops. Remove the tube from the holder by applying pressure against the wings of the Vacutainer holder with the thumb and the index finger. Application of pressure assists in holding the needle steady as tubes are removed and inserted. Invert the tube gently several times after removal to mix blood and additive. Additional mixing per tube type can be performed while the next tubes are filling. Continue to draw blood following the correct order of draw. Needle Removal • The tourniquet must be fully released and the patient’s hand open and relaxed before the needle is removed. • Hold clean a gauze pad in position over the site. Gently and quickly remove needle from arm. • Pressure must be applied to the site, to prevent leakage of blood and possible haematoma formation, as soon as the needle is fully withdrawn, but not before. Pressure is applied firmly to the puncture site using the gauze pad. Assistance from the patient may be sought when possible. • When using a safety-engineered blood collection needle, the safety device is activated at this time. • The patient’s arm should be extended and preferably raised; do not bend the arm, as this increases the risk of haematoma formation. 43 Module 3 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Step 10 Sharps Disposal After successful blood collection, it is important to dispose of the used materials appropriately. The complete assembly should be discarded into a safety box. Remember never to cut, bend, break, burn, or recap needles. Step 11 Mixing of Blood Samples Mixing of the blood sample in the tubes is paramount in maintaining the integrity of the sample quality. The sample is mixed by inversion, as illustrated bel 44 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 3 Why? All tubes contain additive that needs to be mixed with the blood samples. Tubes with an anticoagulant, e.g. EDTA, need to be mixed to ensure the specimen does not clot. How? Holding the tube upright, gently invert 180 degrees and then back. When? This should be done immediately after drawing. Note: There are consequences of failing to mix the samples with the additives in the tube. The samples in tubes with anticoagulant will clot. As a result, the specimen will often need to be redrawn. Order of Draw The order of draw is significant in the prevention of contamination of red top tubes with the EDTA in the analysis of electrolytes. Therefore, the collection of blood should start with the red top Vacutainer followed by the purple top Vacutainer. Order of draw • • Red top tube first to be drawn Purple / lavender top tube second to be drawn Number of inversions Specific specimen tubes have a recommended number of inversions to be done so as to achieve the complete mixing of the sample and additive, as indicated below: • Red: 5X • Purple / lavender: 8X Proper mixing of the samples and the additive ensures that the sample will be fit for analysis. B. Open system In this system, the venipuncture procedure involves the use of needle and syringe. After the needle is in the vein, slowly pull the plunger back to start filling the syringe, and while blood flows into the syringe release the tourniquet. After blood collection, the sample is transferred into the tube by piercing the tube top and vacuum allowed to suck the blood. To ensure that the whole procedure of blood drawing is safe, removal of the needle from the vein should be given attention. This is illustrated below. 45 Module 3 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Transfer of Blood from a Syringe to a Tube • • • • Do not remove the rubber stopper when using evacuated tubes. Place the tube upright in a rack. Slowly pierce the stopper of the evacuated tube with the needle. Allow the tube to fill (without applying pressure to the plunger) until blood flow into the tube ceases. This technique helps to maintain the correct ratio of blood to additive. • Follow the same order for filling tubes as the order of draw for an evacuated system. When transferring blood from a syringe to a tube, do not hold the tube in hand while inserting the needle. Do not recap or bend the needle before disposal. Specimen Labelling This is a very important step after specimen collection. It is done to reduce the risk of specimen misidentification. Label the container after blood is drawn and mix the specimen while the patient is still present. Verify information on the tube labels against the requisition form to ensure all identifiers are accurate. Tubes must not be labelled prior to venipuncture and the patient must not be released before labelling is completed. Post-Venipuncture After specimen collection, the venipuncture site must be taken care of by examining the patient’s arm to see if bleeding has stopped. An adhesive bandage should be applied on the site to fix the gauze pad if the bleeding fails to stop. Instruct the patient to leave the bandage on for a minimum of 15 minutes. For outpatients, advise them not to carry heavy objects with that arm for one hour. Thank the patient for his or her cooperation, as this leaves the patient with a positive feeling. Use gauze instead of cotton. Cotton has fibres that will stick to the venipuncture site, and when removed leads to more bleeding. Dispose of used supplies in a safety box before attending to the next patient. 46 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 3 Removal of Gloves Gloves should be removed without touching the contaminated areas with the hand. • Grasp the wrist of one glove with the opposite gloved hand. • Pull the glove inside out and off the hand. • With the first glove held in the gloved hand, slip the fingers of the non-gloved hand under the wrist of the remaining glove without touching the exterior surfaces. • Pull the glove inside out over the hand so that the first glove ends up inside of the second glove; no exterior glove surfaces are exposed. • Then drop the contaminated gloves into the appropriate waste receptacle. Key Messages 1. Success in blood collection is greatly determined by attention to the safety of the patient, the HCW, and the community. 2. The quality of the specimen is key to proper diagnosis, management of the patient, and the reputation of the facility. 47 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 4 Special Techniques in Specimen Collection by Venipuncture Aim To equip you with knowledge and skills on equipment and techniques used in special conditions in venipuncture Prerequisite Modules Module 1: Importance of Safe Phlebotomy in Patient Care Module 2: Phlebotomy Equipment and Supplies Module 3: Successful Specimen Collection by Venipuncture Objectives By the end of this module you will be able to: 1. 2. 3. 4. Identify equipment used for small and fragile venous access for blood collection List special conditions in venipuncture Identify alternative sites for venous blood collection in special conditions Perform venipuncture using a winged blood collection set Content Outline • Special draw equipment; winged blood collection set; venipuncture in special conditions (paediatrics and geriatrics; burns and thrombosed veins) • Alternative sites; dorsum of the hand and the foot • Use of winged blood collection set: handling and manipulation, needle withdrawal, safety feature activation, disposal, and specimen transfer Notes on Customisation 48 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 4 Special Draw Equipment Introduction This module enables you to discuss those conditions where there are difficulties in blood draw and special care is required while drawing venous blood. Such difficult conditions occur in paediatrics, geriatrics (the elderly), and cancer patients, who may have small and fragile veins for performing venipunture. In this module, the types of equipment used, how to use them, and alternative sites for venous blood collection are discussed. Phlebotomy equipment – special draw While accessing small / fragile veins (paediatric / geriatric patients), use of alternate equipment is recommended. Alternate equipment includes: • Gauge 22/23 needles OR • Winged blood collection set Alternative equipment is required for easing manipulation and reducing the stress exerted on veins (venous collapse). Winged Blood Collection Set with Luer Adapter This figure shows the various parts of a winged blood collection set. Winged Blood Collection Set with Luer Adapter This figure shows the various parts of a winged blood collection set. 49 Module 4 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Needle Sizes and Colour Coding Winged blood collection sets may have different needle sizes and colour coding. The coding is on the wings and is the same as for other needles. The selection of the gauge number depends on the condition or size of the selected vein for venipuncture. The higher the gauge number, the smaller the size of the needle. Green, 21G Blue, 23G Dark blue, 25G Holders When using a winged blood collection set for an evacuated closed collection system, a holder is attached to the luer adapter. Holders are clear and moulded to a standard shape, with wings, or extensions, at the tube end of the holder, which act as a lever against which fingers or thumb can exert pressure to insert or remove the tube while continuing to hold needle steady within the patient’s vein. Advantage: This design prevents the risk of accidental needle stick injury by protecting phlebotomis from the non-patient end of the needle. 50 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 4 Disposal Dispose of holder with the attached needle as one unit. Larger sharps containers may be required to accommodate disposable holders. Winged Blood Collection Set with Syringe A winged blood collection set can also be fitted to the nozzle of a hypodermic syringe attached to other end of the luer slip of the winged set. Remove the luer adapter to fix a syringe to the luer slip (see figure below). The combination is useful while accessing very fragile veins, when the user would like to control the vacuum applied in order to prevent the vein from collapsing. Ensure that the connection is tight, to prevent leakage of vacuum from a loose connection and also formation of froth, which might result in haemolysis. 51 Module 4 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Winged Blood Collection Set with Safety Shield The winged set has a safety shield attached to the needle, so that after use the patient end of the needle is easily and completely retracted into the shield and locked in place. This helps reduce the chance of needle stick injury after use. Other Equipment Needed for Venipuncture Let’s review other equipment needed for performing venipuncture in special conditions. Equipment and Supplies • Tourniquet • Gloves • Antiseptic and cotton • Winged set • Specimen container • Gauze • Adhesive bandage • Safety box 52 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 4 Special Conditions for Venipuncture In addition to paediatric, geriatric, and cancer patients, as mentioned earlier, the following conditions may lead to use of a winged set. • Intravenous lines in both arms • Burned or scarred areas • Cast(s) on arm(s) • Partial or radical mastectomy on one or both sides • Thrombosed veins • Edematous arms In such conditions, blood may need to be drawn from veins in the dorsum of the hand, hence necessitating a special blood draw procedure. Site Selection • • If an IV line is present, blood should be obtained from the opposite arm. In cases where an IV line is in both arms, the following should be done: • Draw from a vein distal to (below) the IV site. • Ask the physician / nurse to turn off the IV for at least 2 minutes. • Place tourniquet between the IV and venipuncture sites. • Perform venipuncture, discarding the first 5 ml of blood. • Indicate the IV solution, arm used, and “drawn below IV”. Alternative Sites Site selection in hand • • • Veins on hands have a narrow diameter; hence it is advantageous to use a small-gauge needle. Use of a winged blood collection set with luer adapter may enhance success and make the procedure less painful. Extra care must be used to anchor these veins to prevent them from rolling. 53 Module 4 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 In case the veins are not visible, wrapping a warm, wet towel around the hand for a few minutes may be considered. This is said to increase blood flow sevenfold, and helps make the veins easier to feel. In case of uncertainty about a vein, tapping the site gently for a few minutes may assist in dilating the veins and making them more prominent. Wrist veins tend to move or roll aside as the needle is inserted, and therefore it is critical to hold the hand such that veins are well anchored. It is preferable to use a winged blood collection set to perform venipuncture on the wrist. Blood Draw from the Foot Foot veins, shown in the figure below, are the last resort for blood collection after the arm veins have been determined to be unsuitable. Due to the risk of complications in some patients (e.g. those with diabetes or coagulopathies), blood collection from foot veins can result in gangrene and thrombosis. Steps in Blood Collection Let’s review the procedure involved in venous blood collection. 54 Module 4 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Patient requisition Patient interaction Standard precautions Selacting equipment Site cleansing Tourniquet application Site selaction Positioning the patient Perform venipuncture Sharps disposal Sample handling/mixing Sample transport The purpose of this is to help you recall the common steps between routine evacuated blood collection and special collection with a winged set. It is important to note the difference in the site of venipuncture. Venipuncture Procedure Using a Winged Blood Collection Set Preparatory Steps 1) 2) 3) 4) 5) 6) 7) Identify and communicate with patient Perform hand hygiene Put on gloves Assemble and arrange required equipment and supplies Position the patient Select a vein Sterilise the area 55 Module 4 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 How to Use a Winged Blood Collection Set • Grasp both wings of the blood collection set using the index finger and thumb of the dominant hand. • Hold the winged blood collection set as shown below, with holder or syringe attached to the non-patient end. While anchoring the veins and keeping skin taut with the thumb of the non-dominant hand, enter the vein at a 10–15° angle. Using non-dominant hand: a) If using evacuated collection, push the tube into the holder using thumb while index and middle fingers grasp wings of the tube holder. b) If using syringe, slowly withdraw the syringe plunger. Blood will now begin to flow. Release the tourniquet. Note: When using evacuated collection method, follow the same order of draw as discussed in the previous module (Module 3). 56 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Withdraw the Needle • • Once the last tube has filled and been withdrawn or the required amount of blood has been withdrawn in the syringe, put a clean gauze pad on the site and apply light pressure using three fingers, as shown. Gently and quickly withdraw the needle and continue applying pressure to the site. Withdraw the needle while grasping the safety shield area with thumb and index finger. With opposite hand, grasp tubing between thumb and index finger. Push the safety shield forwards until the shield locks in place. Discard the complete assembly into an approved safety box. 57 Module 4 Module 4 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Discarding the Blood Collection Set • • Discard the complete assembly without removing the holder, into an approved safety box. Be aware that tubing attached to sharps can recoil and lead to injury – maintain control of both tubing and the device during disposal Transfer of Blood from Syringe into Specimen Container • Remove the cap of the container and gently transfer the specimen into it by pushing the plunger. • Ensure there is no froth formation during blood flow into the container. • Do not overfill. • Replace the container cap. • When evacuated tubes are not used, the phlebotomist needs to remove the cap of the vacuum tube and allow blood to flow on the sides of the walls of the tube while ensuring no froth formation. Avoid pushing the plunger to avoid froth or haemolysis and risk of aerosol formation. Sample Transport • Ensure that the outside of the specimen container is clean and uncontaminated. • Containers should be tightly closed so that the contents do not leak during transportation. • Label and date the container appropriately and complete the requisition form. • Arrange for immediate transportation of the specimen to the laboratory. • During transportation, samples should be placed upright in appropriate racks and placed in appropriate carriers. • Arrange for immediate transportation of the specimen to the laboratory. Notes: • • Delay in sample transportation affects a number of analytes (e.g bilirubin). Criteria should be developed by the laboratory specifying the circumstances under which the processing of a specimen may not be done by the laboratory. Key Message Be aware that tubing attached to sharps can recoil and lead to injury; maintain control of both tubing and device during disposal. 58 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 5 Complications During Specimen Collection by Venipuncture Aim To equip you with knowledge and skills on the management of complications arising from venipuncture Prerequisite Modules Module 3: Successful Specimen Collection by Venipuncture; Module 4: Special Techniques in Specimen Collection by Venipunture Objectives By the end of this module you will be able to: 1) 2) 3) 4) Identify causes of failed venipuncture Outline the corrective actions for failed venipuncture Identify potential patient-related complications arising from venipuncture Describe the management of venipuncture complications Content Outline • • • • Causes of failed venipuncture: improper positioning, rolling of vein, puncture through the vein, needle obstruction Corrective actions for failed venipuncture: troubleshooting failed venipuncture Potential patient complications arising from venipuncture: vomiting, convulsions, excessive bleeding Venipuncture complications management: patient comfort, notification, first aid, following guidelines Notes on Customisation 59 Module 5 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Causes of Failed Venipuncture Failed venipuncture is a condition whereby the blood collection fails. It can also lead to direct complications for the patient. These a) b) complications can be classified into two types: Procedure-related: A problem or situation that makes phlebotomy more difficult and affects the process of drawing blood Patient-related: A problem or situation that happens to the patient during the process of phlebotomy Procedure-Related Complications • • • • • • • • • • Improper positioning of tube Rolling of vein Puncture through vein Needle bevel obstruction Collapsed vein Partially inserted needle Tube pop-off Anticoagulant reflux Accidental arterial puncture Tourniquet not removed Patient-Related Complications • • • • • • Excessive bleeding Petechiae Nausea Vomiting Fainting Convulsions / seizures 60 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 5 Procedure-Related Complications and Their Management Improper Positioning of the Tube This is when the tube is wrongly positioned in the holder, either angulated or with the needle partially piercing the rubber stopper. In a well-positioned tube, the non-patient end of the eclipse needle must penetrate the rubber stopper completely. Tube 1 is correctly / properly inserted into the tube and into the holder. This makes sample collection possible. Tube 2 is incorrectly / improperly inserted, resulting in an incompletely punctured stopper and angulation of the needle in the stopper. This can lead to insufficient quantity collection, underfilling of tubes, and haemolysis of red cells. Tube 3 is incorrectly inserted, since the stopper is partially punctured and therefore cannot obtain the blood sample as desired. Corrective measure: Avoid angulated insertion of the tube or incomplete insertion / puncture through the stopper when inserting the Vacutainer into the holder. Corrective measure: Avoid angulated insertion of the tube or incomplete insertion / puncture through the stopper when inserting the Vacutainer into the holder. 61 Module 5 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Rolling of the Vein This is a condition where the vein moves sideways on attempting to puncture it during phlebotomy. This happens when a vein is not well anchored prior to puncture; it may change position (roll) after or during the process of needle insertion. When a vein rolls, the needle may slip to the side of the vein without penetrating it. When this happens, no sample can be collected since the needle is not in the vein, as shown in this diagram. Corrective measure: • Remove tube from needle holder to preserve vacuum. • Withdraw needle until bevel is just under the skin, anchor vein, and redirect needle into vein. • Reinsert the same tube into the holder. • If there is still no blood flow, remove tourniquet and ensure patient’s hand is open. • Withdraw tube and remove needle from patient’s arm and consider an alternative site, preferably on the opposite arm. Puncture Through Vein This is when the needle penetrates through both walls of the vein. This may happen either when the needle is inserted too fast, or when the holder is not kept steady when tubes are pushed into and removed from the needle. This image shows a needle going through the two walls of the vein. 62 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 5 Corrective measure: • • • • • • • • • Withdraw needle slightly to establish blood flow. If blood flow is restored, take care when removing tube and when inserting and removing subsequent tubes to ensure holder and needle assembly is well anchored (use the wings). Continue but be alert for haematoma. If haematoma begins to form, cease procedure immediately. If blood flow is not restored, then: Remove tourniquet. Ensure patient’s hand is open. Withdraw tube. Remove needle from patient’s arm and apply pressure to the site. Keep arm straight and elevated if possible. Consider alternative site on opposite arm. Remember: Application of pressure to the site at the end of the procedure is key. Needle Bevel Obstruction This is blockage of a needle, and happens occasionally when the bevel of the needle lies against the wall of the vein, preventing free blood flow. 63 Module 5 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 A needle obstructed by the vascular wall (left) needle obstruction leads to difficulties in the flow of blood into the Vacutainer (right) Corrective measure: • • • Pull the needle back slightly. If there is still no blood flow, remove tourniquet and ensure patient’s hand is open. Withdraw tube, and remove needle from patient’s arm. Avoid: • Rotating needle, because this may damage the vessel wall. If you must rotate, do it with care up to a one-quarter turn. • Changing the angle of the needle, because redirection may lead to significant tissue damage. If done, it should be in moderation and with care. It’s good to remove tube while the needle is repositioned, and the same tube can be reinserted in the holder when the vein is accessed. A needle bevel free from the wall of the vein (left) A reestablished blood flow into the tube (right) 64 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 5 Collapsed Vein The vacuum draw of the tube or pressure created by pulling the plunger of a syringe may cause a vein to collapse. Blood flow slows and then stops as the vein collapses. When the vein collapses after the first tube, it may be due to insufficient tourniquet pressure. This can also occur when the tourniquet is applied too close to the puncture site. A vein collapse (left) Blood has already stopped flowing into the tube due to vein collapse (right) Corrective measure: • Ensure needle is in the vein (and that the problem is due to vein collapse). • Experiment with tourniquet pressure (increase or decrease as appropriate). • Try use of a partial-draw tube, since it has a relatively small volume of vacuum compared with the standard Vacutainer. • If blood flow does not resume, remove tube from needle holder, wait a few seconds for blood flow to reestablish, and reinsert tube. If there is still no blood flow, remove tourniquet and ensure the patient’s hand is open before withdrawing tube and needle. Partial-draw tubes are useful in “softening” the draw with small and fragile veins susceptible to collapse. 65 Module 5 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Partially Inserted Needle This is when the bevel of the needle is not totally within the lumen of vein. Blood may leak into the surrounding tissue, causing a haematoma and reduced blood flow into the tube. Haematoma Partially inserted bevel of needle Poor blood flow Corrective measure: • • • • • Remove tube. Release tourniquet and withdraw needle immediately. Apply firm pressure to the site for several minutes and request patient to maintain pressure for a prolonged period if possible, or request assistance from nursing staff or a relative as appropriate. Consider an alternative site on the opposite arm. For haematoma, apply pressure and reassure patient since it will resolve on its own. Tube Pop-Off This is when the tube is ejected, and occurs occasionally during venipuncture where a needle sleeve pushes the tube off the needle slightly and as a result blood flow stops. (left) No blood flows when the tube pops off. (right) Tube (stopper) pushed away by the sleeve of the needle. Corrective measure: Re-advance the tube and hold it in place to reestablish blood flow until the tube is filled. 66 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 5 Reflux of Anticoagulant This is flow of the anticoagulant from the Vacutainer into the vein. If the tube is not properly oriented and the tourniquet is suddenly released, pressure inside the tube may momentarily exceed that in the vein. Blood might then flow back into the patient’s vein (reflux) from the collection tube. This picture shows anticoagulant in the vein (from Vacutainer). Corrective measure: To prevent reflux, the patient’s arm should be maintained in a downwards position to ensure the tube remains below the site and fills from bottom upwards. Tourniquet Not Removed This is when a phlebotomist accidentally forgets to remove the tourniquet after blood starts flowing into the tube. Failure to remove the tourniquet before withdrawing the needle maintains pressure inside vein. Blood can spill out of the vessel once the needle is removed, creating: • Biohazard risk, such as spillage of blood that can lead to contamination • Patient anxiety • Haematoma This image shows a tourniquet forgotten when drawing blood. Always check for any residual tourniquet tension before removing needle. 67 Module 5 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Corrective measures: Tourniquet tension should be reduced or removed when blood begins to flow into the first tube. If not yet removed, make sure it’s loosened or removed completely. Remember that the tourniquet should not be in place for more than one minute during the procedure, since it also compromises quality of the specimen. In case of spillage: • • • Apply pressure to the puncture site after the procedure to reduce risks of bleeding or haematoma formation. Reassure patient and explain that this complication may occur. Decontaminate the surfaces. Patient-Related Complications and Their Management Accidental Arterial Puncture This is accidental puncture to the artery instead of the vein. Arterial blood has a bright red colour and the tube fills very quickly. Management: • • • Remove needle. Apply pressure for at least five minutes. Note on the requisition sheet that the specimen is arterial blood, and inform the lab, since specimen values may be different from those expected. Excessive Patient Bleeding This is when bleeding occurs for longer than five minutes. It can be due to poor procedure, like a tourniquet left in place, or to a bleeding disorder. Management: • Give first aid by applying pressure. If the bleeding does not stop, alert a nurse or any clinician. • Continue applying pressure on the site as long as necessary to stop the bleeding. • Wrap bandage securely around arm over gauze pad. • Leave bandage on the site for at least 15 minutes or until bleeding stops. Petechiae This is the presence of red spots under the skin, usually due to microtrauma or fragile blood vessels below the skin. They may be due to a tourniquet left in place or may represent excessive capillary fragility in some patients. Management: Observe the complication and manage appropriately. You can also investigate for bleeding disorders. Nausea This is an unpleasant feeling preceding vomiting. 68 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 5 Management: • • • • Make patient as comfortable as possible. Instruct patient to breathe deeply and slowly in and out. Apply cold compresses on patient’s forehead. Notify personnel trained in first aid. Vomiting Emesis, or what is known informally as “throwing up.” Management: • Same steps as in nausea. • Give patient an emesis basin, carton, or any other container to vomit in to act as a receptacle. • Give patient water to rinse out mouth. Fainting This is transient loss of consciousness, and can be induced or inflicted by pain. Management: • • • • Notify personnel trained in first aid. If patient is sitting, lay him/her flat or lower his/her head and arms. Loosen tight clothing. Cease procedure. Convulsions / seizures These are abnormal electrical discharges in the brain characterised by involuntary movement of the limbs. Management: • • • • • • Cease procedure immediately. Call for help. Have someone hold pressure to the site. Lower patient to the floor and clear space to prevent injury. Do not restrain patient’s extremities. Notify personnel trained in first aid. Key Message Great care should be taken during and after venipuncture because of patient- and procedure-related complications that may result. 69 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 6 Capillary Blood Collection Aim To equip you with knowledge and skills on capillary blood collection Prerequisite Modules Module 1: Importance of Safe Phlebotomy in Patient Care; Module 2: Phlebotomy Equipment and Supplies Objectives By the end of this module you should be able to: 1. Identify equipment used in capillary blood collection 2. Identify sites for capillary blood collection 3. Perform capillary blood collection Content Outline • • • Equipment: lancets, micro collection tubes, capillary tubes, quick heel safety lancet Sites for capillary blood collection: finger and heel Recommended practices: procedure, complications in capillary blood collection, safety, order of draw Notes on Customisation 70 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 6 Introduction Collection of blood from capillaries is a procedure that involves penetrating the dermis of the skin with a lancet or other sharp device in order to collect a blood specimen. It is also called dermal puncture or skin puncture. Tests • • • commonly performed on capillary blood include: Blood smear - Microscopic assessment of cell morphology - Preparation for malarial parasites HIV - Rapid test - Dried blood spot (DBS) for early infant diagnosis Blood glucose monitoring Key dimensions of successful capillary blood collection are: • Health care worker safety – this can be compromised by blood exposure, sharps injury during the procedure, and injuries due to improper waste disposal • Patient safety and comfort – injuries may occur during a procedure • Specimen quality – the integrity of the specimen can only be maintained by obtaining a specimen that is truly representative of the in vivo status of the patient. The integrity of the specimen can be compromised by poor specimen quality, leading to a wrong result. • Device selection – based on patient’s age and safe penetration depth • Required blood volume: This is determined according to test type. Collection from Children In paediatrics, capillary blood collection is a common practice since children’s veins are small and fragile, unless large volumes of blood are required. Depth of heel and finger sticks: • • • Heel stick should be less than 2.0 mm. Finger stick on children over 12 months should be less than 1.5 mm. Finger stick on children over 8 years should be less than 2.0 mm. Contact with the heel bone (calcaneus) may cause osteomyelitis, which could go undetected for a long period, resulting in serious complications in children. 71 Module 6 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Collection from Adults Skin puncture may be a procedure of choice for blood collection for adults under the following conditions: • • • • • • • • Severe burns – these may result in lack of availability of suitable venipuncture sites Extreme obesity – this makes it difficult to locate / palpate veins Hypercoagulability (thrombotic tendency) – venipuncture could result in serious conse quences for these patients, leading to conditions such as deep vein thrombosis Geriatric or fragile veins – it can be difficult to access / find suitable veins Need to preserve veins for therapy – some patients, such as those on chemotherapy, have superficial, delicate veins that need to be preserved for their therapy Home testing – e.g. glucose testing Apprehensive patients – patients who refuse venous access Point of care testing – within wards (e.g. glucose) Depth of heel and finger sticks: • Heel stick should be less than 2.0 mm. • Finger stick on adults should be less than 2.4 mm. Equipment Used in Capillary Blood Collection In this section you will identify the types of equipment and supplies needed and their applications for successful capillary blood collection. All the equipment and supplies will be reviewed, with emphasis on those that were not discussed in Module 2: Phlebotomy Equipment and Supplies. Equipment used: • Gloves • Lancets • Antiseptic and cotton • Gauze (clean) • Sharps containers • Adhesive bandages • Specimen collection equipment • Micro collection tubes • Slides • Filter paper (for DBS) • Rapid test kits • Capillary tubes 72 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 6 Gloves (see Module 2 for review) Gloves are key supplies in standard infection control. They provide a barrier to the spread of infection. For each patient and procedure, a new pair of gloves should be worn. Gloves are part of personal protective equipment (PPE) against contact with blood during phlebotomy. A good fit is essential; hence one should select the right size. Gloves may not be completely defect-free, and any micropores in the gloves could let contaminants in and hence pose a risk of infection to the health care worker. Gloves should therefore not be washed and reused. Antiseptics (see Module 2 for review) Antiseptics are chemical substances that inhibit or prevent the growth of bacteria. An antiseptic is recommended for use in cleaning the skin prior to drawing blood. The most commonly used antiseptic is 70% isopropyl alcohol. Rapid Test Kits The devices are supplied with defined sample application sites. They are simple to use and require minimum time for test results. Slides These are frequently used for preparation of blood smears. They are made of clear glass. Capillary Tubes These are either plastic- or glass-clad. Plastic-clad capillary tubes are non-sharp, and hence safer for use. Capillary tubes may be with or without anticoagulant. Capillary tubes can be used in blood collection for DBS preparation. 73 Capillaries Module 6 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 • Glass- or plastic-clad capillaries. • Plastic-clad capillaries are safer (non-sharp). • With or without anticoagulant. Micro Collection Tubes These tubes are designed for collection, transportation, and processing of capillary blood from infants, children, and adults with inaccessible veins, and from geriatric and critical care patients. They are colour-coded, similar to evacuated tubes. They do not have vacuum, and hence the caps need to be removed in order to collect the sample. Tubes with anticoagulant require simultaneous mixing to avoid clotting. Tests done using micro collection tubes are similar to those done using evacuated tubes. ection Tubes tubes are designed ansport, and pillary blood. coded similarly to . ollection from: en, adults with eins, andNote: geriatric Micro collection tubes do not have vacuum. e patients. Filter Papers Filter papers are useful devices for sample collection since they provide ease of transport and sample handling. They are used for HIV testing (early infant diagnosis), etc. um. 74 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 6 Cotton This may be supplied as pre-packed swabs or made into cotton balls moistened with 70% isopropyl alcohol just before use. Pre-soaked cotton is not suitable since the active ingredient (alcohol) evaporates, leaving water behind, and the antiseptic property diminishes. Manual Lancets These are for single-use, used for skin punctures, and they are of different shapes and sizes. Their puncture depth depends primarily on the operator and length of needle / blade. Safety Lancets These are single-use devices for skin punctures. They are of different sizes for different depths. The depth should never exceed 2.4 mm. Safety lancets only activate when positioned and pressed against the skin. They facilitate a consistent puncture depth and are permanently retractable. ger Puncture Finger puncture manual lancets punctures. and sizes. epends perator the 75 Module 6 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Finger puncture safety lancets Heel Puncture Safety Lancets These are single-use devices supplied in different sizes. They are automatic and permanently retractable. The blade is used to make a shallow but longer cut, which helps in faster healing as well as better blood flow, since capillaries are concentrated in the top 1 millimetre of the skin. A shallow cut is also less painful, since the top layer of the skin has fewer nerve endings. Use of these devices minimises chances of contact with the bone during the procedure. Heel puncture safety lancets 76 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 6 Sites for Capillary Blood Collection Capillary blood collection is performed from two sites; the heel and the finger. In the case of infants, skin puncture is performed on the heel. For children who have started walking and for adults, it is usually performed on the finger. Heel The heel is the recommended site for capillary blood collection for neonates and infants. The plantar surface of the heel (medial to a line drawn posteriorly from the large toe to the heel or lateral to a line drawn posteriorly from between the fourth and fifth toes to the heel) is the site of choice. Finger The palmar surface on the tip of the third and fourth fingers is the site of choice for capillary blood collection for infants older than one year, as well as for older children and adults. Recommended sites are as indicated in the picture above, because blood capillaries are concentrated on the side of the finger. 77 Module 6 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Capillary Blood Collection Procedure The below graphic shows the steps involved in capillary blood collection. The steps are the same as for venous blood collection with the exception of the tourniquet application step. Patient requisition Patient interaction Standard precautions Selacting equipment Perform venipuncture Site cleansing Site selaction Positioning the patient Sharps disposal Sample handling/mixing Patient Requisition, Patient Interaction, and Standard Precautions (Steps 1–3) Steps 1–3 are important in blood collection procedure. However, these steps have been adequately covered in Module 3: Successful Specimen Collection by Venipuncture. In capillary blood collection procedure, therefore, we shall only deal with the equipment selection step through the sample transport step. Step 4: Selecting Equipment After verifying the information on the requisition form, it is important to select micro collection devices and assemble them according to the test to be done before starting the procedure. Step 5: Positioning the Patient Adults and older children should sit with their arm flat on a surface. Children can be restrained by either having them sit beside a parent or on the parent’s lap. The parent should wrap their arm around the child and over the arm that is not being used. The parent could also restrain the child by lying down on a couch/bed as shown. 78 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 6 Positioning for the heel prick Step 6: Site Selection After site selection, it may be necessary to warm the puncture site to increase the blood flow. Warming is known to increase blood flow sevenfold. This can be done with a warm, moist towel or warming device for 3 to 5 minutes. 79 Module 6 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Step 7: Site Cleansing The skin puncture site must be cleaned with 70% isopropyl alcohol as pre-packed swabs or cotton moistened with the antiseptic at the time of use. Allow the area to air-dry so that the antiseptic action of the alcohol can take effect. Residual alcohol causes rapid haemolysis and can adversely effect some test results (e.g. blood glucose determination). Betadine (povidine iodine) must not be used to clean and disinfect skin puncture sites: blood contaminated with betadine may have falsely elevated levels of potassium, phosphorus, or uric acid. Clean the area starting from the intended puncture site and work outwards to prevent contaminating the area. Step 8: Perform Skin Puncture – Finger When carrying out this procedure, it is appropriate to inform adult patients about imminent pain. Hold the finger and firmly place a new sterile lancet at the selected site, and then orient the lancet across the fingerprint groves to help in drop formation. If using a self-retracting safety lancet, activate it; or if using a manual lancet, perform a single puncture in one smooth motion. across X parallel Skin Puncture – Heel During heel puncture, hold the foot in a firm grip with the thumb placed below the puncture site and index finger placed over the arch. Hold and orient the lancet on the intended puncture site at a 90-degree angle / perpendicular to the length of the foot. Firmly and completely depress the trigger of the safety lancet or perform a single puncture with one smooth motion if using a manual lancet. The following precautions should be observed during heel puncture procedure: • Do not puncture deeper than 2.0 mm to avoid contact with the bone. • Do not puncture through previous punctures. • Do not puncture outside the medial and lateral aspects of the heel, as previously described. • Do not puncture the posterior curvature or the arch of the heel. • Do not puncture areas of the foot other than the heel. 80 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 6 Blood Collection from Finger or Heel After successful puncture, wipe out the first drop of blood with a clean, dry gauze pad, as it contains an excess of tissue fluid which would dilute the sample, thereby affecting the analyte results. Collect the sample beginning with the second drop, using the appropriate device according to the test being performed (e.g. slides, capillary tubes, rapid test, micro collection tubes, filter papers). Holding the puncture site downwards, gently apply intermittent pressure to the surrounding tissue to enhance blood flow. Avoid “milking” or scraping since this can cause sample dilution with tissue fluids. Continue until the desired volume of blood has been collected. Note: Before starting the sample collection procedure, ensure the waste containers are within reach. Blood Collection on Filter Papers (DBS Preparation) Dried blood spots (DBS) are prepared by collecting whole blood on designated filter papers positioned in a drying rack. Label the filter paper appropriately before using. Using a capillary tube, , collect sample from the puncture site and put two drops on each circle. NAME: DATE: 81 Module 6 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 When preparing DBS, avoid the following: • • • • • Oversaturated spots Scratches on spots Scattered spots More than one layer Touching or smearing the blood spot Drying After preparation, allow the specimen to fully air-dry horizontally overnight at room temperature. This ensures complete drying, as demonstrated by a chocolate-brown colour. While • • • • drying avoid the following: Direct sunlight Dust Heat Stacking or touching other surfaces Post–Skin Puncture Procedure When blood collection is complete, it is the role of the phlebotomist to ensure bleeding from the site of puncture is stopped. This is achieved by applying clean, dry gauze at the bleeding point and asking the patient to apply pressure for a short time. Do not apply an adhesive bandage on children below two years, since this may irritate infant skin, or the infant may remove it, put the bandage in the mouth, or swallow it. Ensure all specimens are labelled immediately after the draw. Ensure proper disposal of all waste. Key Messages 1) 2) 3) 4) 5) The first drop of blood must be discarded to avoid specimen dilution by tissue fluid. Never “milk” or scrape the puncture site. Mix anticoagulated micro collection tubes continuously during collection. Never exceed the specified puncture depth to avoid osteomyelitis. Follow recommended order of draw for micro collection tubes. 82 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 7 Safety and Infection Control Aim To equip you with knowledge, skills, and attitudes on infection prevention and control practices Prerequisite Modules Module 3: Successful Specimen Collection by Venipuncture Module 4: Special Techniques in Specimen Collection by Venipuncture Module 6: Capillary Blood Collection Objectives By the end of this module you will be able to: 1. 2. 3. 4. List infectious agents potentially transmissible during blood collection Describe risk factors of blood-borne pathogens during blood specimen collection Outline standard precautions in the workplace Describe the waste management process Content • Infectious agents: viral, bacterial, fungal, and protozoan • Risk factors: non-intact skin, needle stick and sharps injuries, splashes on mucous membrane • Appropriate use of PPE and safe work practices • Waste management: proper waste segregation, containment, handling, storage, transport, treatment or destruction and disposal of sharps Notes on Customisation 83 Module 7 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Infectious Agents These are pathogenic microorganisms that when present in human blood can cause diseases. HCWs are at a higher risk due to occupational exposure to blood-borne pathogens. Exposure can occur through various types of contact: • Needle stick injuries (NSIs) • Mucous membrane • Non-intact skin HCWs must therefore take precautions while collecting or handling blood and other body fluids to avoid coming into contact with infectious agents. These infectious agents are classified into the following categories: viral, bacterial, fungal, and protozoan. Diseases That Can Be Contracted through NSI The table below gives a breakdown of the pathogens which can be contracted through NSIs. Viral Infections Bacterial Infections Fungal Infections Hepatitis B Brucella abortus Blastomyces dermatidis Hepatitis C Corynebacterium diptheriae Cryptococcus neoformans Hepatitis G Neisseria gonorrheae Sporotrichum schenkii Human immunodeficiency virus Leptospira icterohaemorrhagiae Simian immunodeficiency virus Mycobacterium marinum Protozoal infections Herpes simiae Mycoplasma caviae Plasmodium falciparum Herpes simplex Orientia tsutsugamushi Toxoplasma gondii Herpes zoster Rickettsia rickettsii Ebola/Marburg Staphylococcus aureus Dengue Streptococcus pyogenes Creutzfeldt-Jakob disease Treponema pallidum Mycobacterium tuberculosis Risk Factors Risk of infection due to sharps injury The data below showan estimated risk burden attributed to contaminated sharps injuries among health workers. The data emphasise that not only HIV poses a high risk with exposure to contaminated sharps but also HBV and HCV, the incidence of which are both much higher. 84 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Virus HBV HCV HIV Module 7 NSI risk burden 23–62% 0–7% 0.3–0.5% Risk Factors for HIV/HCV/ HBV Transmission after Percutaneous Exposure Several factors increase one’s susceptibility to infection from an NSI. • Deep injury • Injury with device visibly contaminated with blood • Procedures involving placement of device in patient’s artery or vein • Hollow-bore needles • High viral load in source patient • Failure to take or complete post-exposure prophylaxis for HIV • Failure to get vaccination against HBV Appropriate Use of PPE and Safe Work Practices These are procedures/practiceswhich prevent thetransfer of pathogenic microorganisms from the source to the HCW, general population, and environment. Standard precautions • Safe and proper use of equipment • Effective hand hygiene procedures • Immunisations, e.g. hepatitis B • Proper decontamination of surfaces and instruments • Keeping safety boxes at arm’s length • Labelling of all biohazard materials • Use of gloves during blood drawing • Use of gowns, masks, respirators, and other PPE wherever indicated • Use of closed-toe shoes or boots Note: All samples should be considered potentially infectious. Hand Hygiene This is the act of keeping hands free from contamination by washing/sanitising them frequently to prevent the spread of infection. Situations when hand washing/sanitising is required: • Before and after each patient contact • Between unrelated procedures (e.g. wound care, blood draw) • Before putting on gloves and after taking them off • Before and after going to the washroom • Whenever hands become visibly or knowingly contaminated 85 Module 7 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Hand hygiene can be performed by: • Hand washing using soap and water • Using hand gel / sanitiser Hand washing with soap and water is the most important means of preventing and controlling the spread of infection. Sharps Safety Practices These • • • • • • • practices help ensure proper handling of sharps to prevent NSIs. Be prepared before beginning a procedure. Organise equipment at the point of use. Make sure the workplace has adequate lighting. Keep sharps pointed away from the user. Keep safety boxes within arm’s length. Assess a patient’s ability to cooperate and get help if necessary. Ask patient to avoid sudden movement. Be Aware During a Procedure • • • • • • • • Maintain visual contact with sharps. Be aware of sharps nearby. Control location of sharps to avoid injury to yourself and others. Do not pass exposed sharps from one person to another. Use predetermined “neutral zones” for placing / retrieving sharps. If using devices with safety features: Activate as soon as procedure is completed. Observe audible or visual cues that confirm the feature is locked in place. Failure to follow the above precautions exposes the patient as well as other HCWs to accidental needle stick injury. The safety box should be kept at arm’s length so that sharps can be disposed of immediately after use; do not leave them “laying around” as pictured below. 86 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 7 Dispose with Care • Be accountable for the sharps you use. • Check procedure trays, waste materials, and bedding for exposed sharps before handling. • Look for sharps / equipment left behind inadvertently. • Inspect safety box visually (do not overfill). • Always keep hands behind sharps. • Never put hands or fingers into the sharps container. • Use mechanical device if you cannot safely pick up sharps by hand. • If disposing of sharps with attached tubing: • Be aware that tubing attached to sharps can recoil and lead to injury. • Maintain control of both tubing and the device. Proper Use of Safety Boxes An overfilled safety box (below) with protruding sharps poses a risk of sharps injury, especially to the next person depositing sharps in the container, even when closing it before disposal. Usually, the safety boxes have a fill line beyond which it should not be filled. If using a safety box that is not transparent, the user should check the fill level through the opening of the box with great care while maintaining a safe distance. Note: Replace safety boxes before they become overfilled. 87 Module 7 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Handling Broken Samples The picture below illustrates how the health care worker can most safely handle broken sample containers on the floor. Disposal It is important that proper segregation of waste is maintained in the department. This prevents exposing the person responsible for collecting waste (non-sharps) to possible sharps injury, as shown below. Sharps in a non-sharps waste bin could pose a serious risk of needle stick injury to workers responsible for handling waste. This form of sharps injury could be even more serious, because of lack of awareness in personnel handling waste as well as the difficulty in linking the sharp to the patient on which it was used. 88 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Waste Management Waste management is the proper handling, treatment, and disposal of medical wastes. Purpose of waste management: • To protect people who handle waste items from accidental injury • To prevent the spread of infection to HCWs who handle waste • To prevent the spread of infection to the local community • To safely dispose of hazardous material Measures to Reduce the Risk of Infection from Medical Waste • • • • • • • Segregate waste at the source. Use PPE when handling medical wastes. Handle sharps with care. Do not sort through waste. Keep facility clean inside and outside. Have knowledge in first aid. Get fully immunised against tetanus and hepatitis B. In case of injury, go for immediate evaluation for PEP. Key Steps in Waste Management 89 Module 7 Module 7 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Waste Segregation This is the process of separating generated medical wastes into their appropriate bins before disposal. It should be done at the point of generation by type and category, as follows: • • • • Non-infectious – disposed in the black waste bin (e.g. papers, food remains) Infectious – disposed in the yellow bin (e.g. used gloves, cotton, gauze) Highly infectious – disposed in the red bin (e.g. body fluids, such as blood, aspirates, used culture media) Sharps (highly infectious) – disposed in safety boxes Containment This is the safe method of managing segregated waste in an orderly manner to prevent cluttering. The purpose is to reduce or eliminate exposure of HCWs and the environment to potentially hazardous agents. Handling and Storage This is the safe method of ensuring waste is kept in safe and designated rooms awaiting collection to the disposal site. Labelling should be done according to type and category. Transportation This is the transporting of medical waste to disposal sites by appropriate means. There are two types of disposal sites: • • On-site: within the facility by using a wheelbarrow, waste trolley, or hand cart Off-site: outside the facility by using a dedicated waste collection vehicle; a licence must be obtained from the relevant authority (e.g. NEMA or local authority). Each waste category should be transported separately. Precautions to follow during transport: • Keep boxes upright. • Keep safety boxes dry. • Avoid direct contact of safety boxes with other medical wastes or medical supplies in the same vehicle. • After transport, clean vehicle surfaces with disinfectant. 90 Module 7 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Treatment Medical waste treatment is a process that renders medical waste harmless to the health care worker, general population, and the environment. The commonly used methods are: • • • Burning and burying Incineration Autoclave (limited to laboratory specimens) Waste Disposal Incinerator (top and bottom) Waste shredders Open disposal and burning Key Message It is not just about numbers 91 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 8 Occupational Exposure Aim To equip you with knowledge and skills to manage occupational exposure to blood and body fluids Prerequisite Modules Module Module Module Module 3: 4: 6: 7: Successful Specimen Collection by Venipuncture Special Techniques in Specimen Collection by Venipuncture Capillary Blood Collection Safety and Infection Control Objectives By the end of this module you should be able to: 1. 2. 3. 4. 5. Define terminologies in occupational exposure Describe the risk of transmission of HIV and hepatitis after occupational exposure Identify the devices causing percutaneous injuries Outline PEP management Outline institutional policy in relation to occupational exposure Content • Terminologies: occupational exposure and PEP • Risk of transmission of different viruses following accidental needle stick injury • Devices involved in percutaneous injuries or exposure: hypodermic needles, sutures, scalpels, etc. • PEP management procedures: goals, immediate measures, first aid, evaluation for PEP, PEP regimens, administering PEP, monitoring drug interactions, side effects, and follow-up • Institutional policies such as having a PEP plan, appropriate use of the PEP register, and advocating for hepatitis B vaccination for all health care workers Notes on Customisation 92 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 8 Terminology 1. 2. 3. Occupational exposure to HIV refers to an accidental exposure to blood or body fluids with a potential risk of transmitting HIV to HCWs during performance of their duties. Post-exposure prophylaxis (PEP) refers to the preventive management given to minimise the risk of infection following potential exposure to HIV. Non-occupational exposure refers to exposure to potential blood-borne infections (HIV, HBV, HCV) outside the work setting, e.g. sexual assault / rape / sodomy. Risk of Transmission Although the main focus is HIV, it is also important to note that hepatitis B and C have a higher risk of transmission than HIV. It is important to note that the consequences of viral infections are expensive and associated with many complications and that there is no effective management for viral hepatitis. Risk of transmission of different viruses following accidental NSI Hepatitis B 6–30% Hepatitis C 1.8% HIV0.3% Exposure Risk for HIV This table shows the comparative risk of acquiring HIV through different portals of entry. The risk is highest for percutaneous exposure and lowest for non-intact skin. Percutaneous0.3% Mucous membrane0.1% Non-intact skin< 0.1% Devices that May Cause Percutaneous Injuries Devices that are associated with percutaneous injuries are: • • • • • Hypodermic needles Blood collection needles Suture needles Needles used in IV delivery systems Scalpels 93 Module 8 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Factors Increasing the Risk of Sharps Injuries Past studies have shown that sharps injuries are often associated with the activities outlined below. • The risk of sharps injuries is highest when sharps are not handled and disposed of correctly. • Injuries can also occur when recapping needles or other devices. • Injuries can occur when transferring a body fluid between containers. • Injury can be caused by failing to dispose of used needles or other devices properly in puncture-resistant sharps containers. Goal of Post-Exposure Prophylaxis The ultimate goal of PEP is to maximally suppress viral replication that may occur, and to shift the biological advantage to the host cellular immune system to prevent or stop early infection. First Aid • • • • • • For percutaneous exposure, wash needle stick injuries and cuts with soap and copious amount of free-flowing water. For non-intact skin exposure, wash with soap and water as explained above. For mucous membrane exposure, irrigate the nose, mouth, or skin with water. Avoid use of soap since it can cause irritation and increase risks of contracting HIV infection. Irrigate eyes with copious amount of clean water or sterile saline. Report exposure to the immediate supervisor. Ensure initiation of the first dose of PEP as soon as possible. PEP should be initiated preferably within 2 hours and not later than 72 hours post-exposure to optimise its benefit. Evaluation of Exposure and Source of Exposure • Evaluate the type of body substance (e.g. blood, urine, pus) involved. • Evaluate the severity of exposure. • Establish baseline / initial HIV and HBV status of the source of exposure (patient). • Establish baseline / initial HIV and HBV status of the exposed HCW. • Determine appropriate PEP regimen depending on the severity of exposure. Management and Follow-Up The principles of PEP management are: • • • Counselling and provision of PEP drugs Monitoring and managing PEP toxicity 2 weeks after starting PEP HIV testing at 6 weeks, 3 months, and 6 months Note: Efavirenz is teratogenic; hence, test to rule out pregnancy in women. 94 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 8 The tolerability of HIV PEP in HCWs is affected by the side effects listed in the graph below. Incidence of common side effects Duration of PEP Therapy • A four-week course of therapy equivalent to 28 days • Adherence is taking medicines as prescribed, taking into consideration the timing and the actual dosages. Access to Therapy Access to the full course of PEP drugs must be ensured for all HCWs. • A 3-day supply should be available • 24 hour/7-day availability of services must be ensured 95 Module 8 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Institutional Policies Different institutions have different strategies on the provision of post-exposure management. The following are recommended guidelines: • • • • • • • • • • • • • • • Have a plan for immediate provision of PEP. Have a plan for counselling HCWs: Protect HCW’s confidentiality about exposure, treatment, and test results. Acknowledge and be prepared to address fears. Review the HCW in 2–3 days to answer questions and clarify issues. Review medication frequently for: Possible toxicities Interactions Adherence Arrange for referral to an HIV comprehensive care clinic. Provide contacts for questions. Provide counselling about: Sexual and reproductive issues Breastfeeding Avoid donation of blood, plasma, organs, tissue, or semen. Recommendations for Hepatitis • • • • • All HCWs should be immunised against HBV For unimmunised HCWs, give prophylactic HBIG (hepatitis B immunoglobulin) and initiate the vaccine series. There is no effective prophylaxis against hepatitis C, and administration of immuno globulin and antiviral agents is NOT recommended. Determine status of source and establish baseline serology of employee and repeat testing at 4–6 months post-exposure. Early treatment is advised if infection occurs. Key Messages 1) 2) 3) More and more patients with HIV infection continue to seek treatment and a greater number of NSIs are likely to occur. The risk of HIV transmission is low. The availability of PEP should not preclude taking standard precautions 96 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Module 9 Inventory Management Aim To equip you with knowledge and skills on inventory management Prerequisite Module Module 2: Phlebotomy Equipment and Supplies Objectives By the end of this module you will be able to: 1) 2) 3) Define terms used in inventory management Identify tools used in inventory management Outline steps in inventory management Content • Definition of terms: inventory, inventory management • Inventory tools (S11, S12, stock card) • Inventory process: requesting, receiving, storage, issuing/distribution, use, and reporting • Storage: general guidelines for storage practices Notes on Customisation 97 Module 9 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Definitions and Tools Inventory: refers to a list of goods / materials held in stock in an institution at a given time. Inventory management: refers to the process of ordering, receiving, storing, distributing, and issuing of items and reporting on consumption. The goal of efficient inventory management is to maintain a steady supply of stock to the operating units (e.g. benches, wards) while minimising costs associated with overstocking and understocking. S11 Form This form is used to order supplies from the main store to the departmental store (e.g. laboratory store). FORM S11 Serial No.......................................... REPUBLIC OF KENYA COUNTER REQUISITION AND ISSUE VOUCHER Ministry .................................................................. Dept Branch................................................ Unit.......................................................... To (Issue Point)........................................................................................................................................................................................................................................................... Please issue the store linked below to (Point of use)............................................................................................................................................... Code No. Item Description Account No: ......................................................... Requisitioning officer .......................................... Issued by: ............................................................ Received by:.............................................. Unit of Issue Quantity Required Quantity Issued Designation: ......................................................... Signature: ............................................................ Designation: ............................................................ 98 Value Remarks/Perpose Date: .................................................................. Sign: .................................................................. Date: .................................................................. Sign: .................................................................. Module 9 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Stock and Bin Card This card is used to enter all supplies ordered from the main store to the departmental store. MINISTRY OF HEALTH Serial No.......................... Laboratory Stock Card District................................................................ Commodity name and Description Name of Facility....................................................... Unit of issue Item Code Average Monthly Consumption Date Received From RECEIPTS Doc. Quant. No. Storage requirements Minimum Level Batch No. Expiry Loc. Name Maximum Level DISBURSEMENTS/ISSUES Doc. Name No. Quant. Dest. Doc. No. - Document Number, Quant. - Quantity, Loc. - Location, Dest. - Destination STOCK Unit Balance Value Total Value Balance C/F.......................... to Card No. ................. Top-Up Form This is used to order from the departmental store to the point of use. MINISTRY OF HEALTH Serial No.......................... NATIONAL PUBLIC HEALTH LABORATORY SERVICES LABORATORY TOP-UP FORM Name of Facility.......................................................... Date Commodity Department/Section.................................................................. Current Tests done Order Issue UnityofIssue Balance Quantity Quantity 99 Issued by Name Received by Sign Name Sign Remarks Module 9 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 S12 Form This is used to order supplies between facilities. Steps in Inventory Management Requesting / Reporting/ using data reordering supplies Use, tracking/ monitoring Receiving supplies Storage bulk/ point of use General Guidelines for Good Storage Practices • • • • • • • • • • • Provide appropriate space and security for stored stock. Provide safe and orderly arrangement of stock in the store. Maintain correct storage conditions to safeguard quality. Good stock control and rotation: practice first expiry, first out (FEFO) and first in, first out (FIFO). Label clearly all reagents and chemicals with their name, date of preparation, and hazard symbol. Store chemicals, reagents, and other supplies correctly zoned, making sure incompatible chemicals are not stored together. Secure storage areas, e.g. for expensive or dangerous items. Maintain the correct temperature conditions for items, especially reagents. Conduct regular physical stock counts and record them. Develop and implement standard operating procedures (SOPs) for storage of supplies. Maintain updated stock cards and track expiry dates of supplies using the expiry tracking chart. 100 Module 9 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 Expiry Tracking Chart Serial No.......................... MINISTRY OF HEALTH Expiry Tracking Chart for Laboratory Reagents and Consumables Commodity Batch No. Ecpiry Date Year: Year: Year: Stock Count This is physically counting each item in the store. It is done at the beginning of each month by a designated person. Note: All items must be accounted for. Everything that comes in and goes out must be recorded. Key Messages 1) 2) 3) 4) 5) Order supplies that you can use before their expiry. Always inspect a new shipment before accepting it. Maintain an adequate inventory at all times to ensure uninterrupted supply. Ensure that the inventory is tracked at all times by ensuring that the tools are well filled in / used and reported in a timely manner. Ensure that all items in the inventory are accounted for and recorded. 101 Module 9 Safe Phlebotomy Training for Health Care Workers in Kenya: Participant’s Manual, 2013 ADDITIONAL RESOURCES Becton, Dickinson. Product catalogue 2006. Franklin Lakes, NJ, USA: Becton, Dickinson, 2006. Becton, Dickinson. “Vacutainer Order of Draw and Mixing Guidelines 2007.” Franklin Lakes, NJ, USA: Becton, Dickinson, 2007. Becton, Dickinson. Product catalogue 2010. Franklin Lakes, NJ, USA: Becton, Dickinson, 2010. Becton, Dickinson (BD) Diagnostics. “Successful Specimen Collection: Fingersticks: Recommended Procedure with the BD MicrotainerMicrocollection System. Job aid. Franklin Lakes, NJ, USA: BD Diagnostics, 2008. http://www.bd.com/vacutainer/labnotes/pdf/Volume20Number1_wallchart.pdf (accessed August 2013). Government of Kenya (GOK).Kenya National Curriculum for Laboratory Technologists Refresher Training in Integrated Disease Surveillance and Response (IDSR), 2003–2004. Nairobi: GOK, 2004. Idaho Division of Professional-Technical Education (PTE).Instructor’s Guide for Training Phlebotomists.Boise, Idaho, USA; Idaho Division of PTE, 2006. http://www.pte.idaho.gov/pdf/health/ curriculum/phlebotomycurriculum.pdf (accessed August 2013). Infection Control Resource: Prevention Strategies for IC Practitioners and Professional Nurses.4 (1). http://www.infectioncontrolresource.org/Past_Issues/IC13.pdf (accessed August 2013). Ministry of Public Health & Sanitation, and Ministry of Medical Services.Safe Phlebotomy Training Manuals.Revised 2011. Nairobi: Government of Kenya, 2011. National AIDS and STI Control Programme (NASCOP), Ministry of Health, Kenya. Kenya AIDS Indicator Survey 2012: Preliminary Report. Nairobi: NASCOP, 2013. National AIDS and STI Control Programme (NASCOP), Ministry of Health, Kenya. Kenya AIDS Indicator Survey 2007: Final Report. Nairobi: NASCOP, 2009. Prüss-Ustün A, Rapiti E, Hutin Y. Estimation of the Global Burden of Disease Attributable to Contaminated Sharps Injuries Among Health-Care Workers. Am. J. Ind. Med. 2005; 48(6):482– 490. US Centers for Disease Control and Prevention (CDC).Strengthening Laboratory Systems in Support of ART Services Training Curriculum: June 2006. Nairobi: CDC-Kenya, 2006. World Health Organization.“Practical Guidance on Venepuncture for Laboratory Testing.”WHO Health Technologies e-Documentation Centre.Geneva: WHO, 2010. http://hinfo.humaninfo.ro/ gsdl/healthtechdocs/documents/s17247e/s17247e.pdf (accessed August 2013). 102