CHAPTER 5: IMPERFECTIONS IN SOLIDS Imperfections in Solids
advertisement
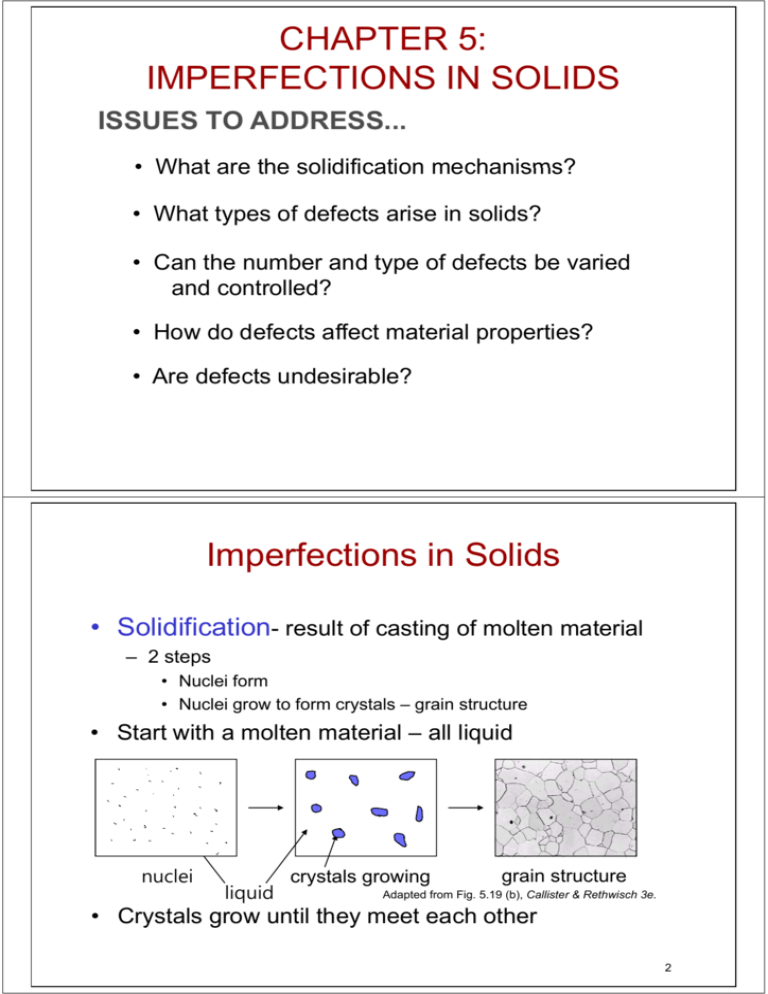
CHAPTER 5: IMPERFECTIONS IN SOLIDS ISSUES TO ADDRESS ADDRESS... • What are the solidification mechanisms? • What types yp of defects arise in solids? • Can the number and type of defects be varied and controlled? • How do defects affect material properties? • Are defects undesirable? Imperfections in Solids • Solidification- result of casting of molten material – 2 steps p • Nuclei form • Nuclei grow to form crystals – grain structure • Start with a molten material – all liquid nuclei liquid crystals growing grain structure Adapted from Fig. 5.19 (b), Callister & Rethwisch 3e. • Crystals grow until they meet each other 2 Polycrystalline Materials Grain Boundaries • regions between crystals • transition from lattice of one region i tto th thatt off the th other • slightly disordered • low density in grain boundaries – high mobility – high diffusivity – high chemical reactivity Adapted from Fig Fig. 5 5.12, 12 Callister & Rethwisch 3e. 3 Solidification Grains can be - equiaxed (roughly same size in all directions) - columnar (elongated grains) ~ 8 cm heat flow Columnar in area with less undercooling Adapted from Fig. 5.17, Callister & Rethwisch 3e. Shell of equiaxed grains due to rapid cooling (greater ΔT) near wallll Grain Refiner - added to make smaller smaller, more uniform uniform, equiaxed grains grains. 4 Imperfections in Solids There is no such thing as a perfect crystal. • What are these imperfections? y are they y important? p • Why Many of the important properties of materials are due to the presence of imperfections. 5 Types of Imperfections • Vacancy atoms • Interstitial atoms • Substitutional atoms Point defects • Dislocations Line defects • Grain Boundaries Area defects 6 Point Defects in Metals • Vacancies: -vacant vacant atomic sites in a structure structure. Vacancy distortion of planes • Self-Interstitials: -"extra" atoms positioned between atomic sites. selflf interstitial distortion of planes 7 Equilibrium Concentration: Point Defects • Equilibrium concentration varies with temperature! No. of defects No. of potential defect sites Activation energy ⎛ Q Nv = exp ⎜⎜ − v ⎝ kT N ⎞ ÷ ÷ ⎠ Temperature Boltzmann's constant -23 23 (1.38 x 10 J/atom-K) -5 (8.62 x 10 eV/atom-K) Each lattice site is a potential vacancy site 8 Measuring Activation Energy • We can get Qv from an experiment. ⎛ −Q Nv v = exp ⎜⎜ ⎝ kT N • Measure this... • Replot it... Nv ln N Nv N ⎞ ÷ ÷ ⎠ slope -Qv /k exponential dependence! T 1/T defect concentration 9 Estimating Vacancy Concentration • Find the equil. # of vacancies in 1 m3 of Cu at 1000°C. • Given: ρ = 8.4 g /cm 3 A Cu = 63.5 g/mol Qv = 0.9 eV/atom NA = 6.02 x 1023 atoms/mol 0 9 eV/atom 0.9 ⎛ ⎞ −Q v ÷ Nv = ⎜ -4 ÷ exp p⎜ ⎝ kT ⎠ = 2.7 x 10 N For 1 m3 , N= ρ x NA A Cu 1273 K 8 62 x 10-5 eV/atom 8.62 eV/atom-K K x 1 m3 = 8.0 x 1028 sites • Answer: Nv = (2.7 (2 7 x 10-44)(8.0 )(8 0 x 1028) sites it = 2.2 2 2 x 1025 vacancies i 10 Observing Equilibrium Vacancy Conc Conc. • Low energy electron microscope i view i off a (110) surface of NiAl. • Increasing temperature causes surface island of g atoms to grow. • Why? The equil. vacancy conc. increases via atom motion from the crystal to the surface, where they join the island. island Island grows/shrinks to maintain q vancancy y conc. in the bulk. equil. Reprinted with permission from Nature (K.F. McCarty, J A Nobel, J.A. Nobel and N.C. N C Bartelt, Bartelt "Vacancies Vacancies in Solids and the Stability of Surface Morphology", Nature, Vol. 412, pp. 622-625 (2001). Image is 5.75 μm by 5.75 μm.) Copyright (2001) Macmillan Publishers Ltd Publishers, Ltd. 11 Point Defects in Ceramics (i) • Vacancies -- vacancies acancies e exist ist in ceramics for both cations and anions • Interstitials -- interstitials exist for cations -- interstitials are not normally observed for anions because anions are large relative to the interstitial sites Cation Interstitial Cation Vacancy Anion Vacancy Adapted from Fig. 5.2, Callister & R th i h 3 Rethwisch 3e. (Fig. (Fi 5 5.2 2 iis ffrom W.G. Moffatt, G.W. Pearsall, and J. Wulff, The Structure and Properties of Materials, Vol. 1, Structure John Wiley and Sons Structure, Sons, Inc., p. 78.) 12 Point Defects in Ceramics (ii) • Frenkel Defect -- a cation vacancy-cation interstitial pair. • Shottky Defect -- a paired set of cation and anion vacancies. Shottky Defect: Adapted from Fig. 5.3, Callister & Rethwisch 3e. (Fig. 5.3 is from W.G. Moffatt, G.W. Pearsall, and J Wulff, J. W lff The Th Structure St t and d Properties of Materials, Vol. 1, Structure, John Wiley and Sons, Inc., p. 78.) Frenkel Defect • Equilibrium concentration of defects ∝ e −QD /kT 13 Imperfections in Metals (i) Two outcomes if impurity (B) added to host (A): • Solid S lid solution l ti off B in i A (i.e., (i random d di dist. t off point i td defects) f t ) OR Substitutional solid soln. ( (e.g., C in Cu i Ni) Interstitial solid soln. ( (e.g., C in i Fe) F ) • Solid solution of B in A plus particles of a new phase h ((usually ll ffor a llarger amountt off B) Second phase particle -- different composition -- often different structure. 14 Imperfections in Metals (ii) Conditions C diti ffor substitutional b tit ti l solid lid solution l ti (S (S.S.) S) • W. Hume – Rothery y rule – 1. Δr (atomic radius) < 15% – 2. 2 Proximity in periodic table • i.e., similar electronegativities –3 3. Same crystal structure for pure metals – 4. Valency • All else being equal, a metal will have a greater tendency to dissolve a metal of higher valency than one of lower valency 15 Imperfections in Metals (iii) Application of Hume–Rothery Hume Rothery rules – Solid Solutions Element Atomic Crystal ElectroRadius Structure S (nm) 1. Would ou d you p predict ed ct more Al or Ag to dissolve in Zn? 2. More Zn or Al in Cu? Cu C H O Ag Al Co Cr Fe Ni Pd Zn 0.1278 0.071 0.046 0.060 0.1445 0.1431 0.1253 0.1249 0.1241 0.1246 0.1376 0.1332 Valence negativity FCC 1.9 +2 FCC FCC HCP BCC BCC FCC FCC HCP 1.9 1.5 1.8 1.6 1.8 1.8 2.2 1.6 +1 +3 +2 +3 +2 +2 +2 +2 Table on p. 159, Callister & Rethwisch 3e. 16 Imperfections in Ceramics • Electroneutrality (charge balance) must be maintained when h iimpurities iti are presentt Cl • Ex: NaCl Na + • Substitutional cation impurity cation vacancy Ca 2+ Na + Na + without impurity Ca 2+ impurity • Substitutional anion impurity Ca 2+ with impurity anion vacancy O2- without impurity Cl Cl O2- impurity with impurity 17 Point Defects in Polymers • Defects due in part to chain packing errors and impurities such as chain h i ends d and d side id chains h i Adapted p from Fig. g 5.7,, Callister & Rethwisch 3e. Adapted from Fig. 5.7, Callister & Rethwisch 3e. 18 Impurities in Solids • Specification S ifi ti off composition iti – weight percent C1 = m1 x 100 m1 + m2 m1 = mass of component 1 – atom percent C1' = n m1 x 100 n m1 + n m 2 nm1 = number of moles of component 1 19