2012 Symposium on Human Factors and Ergonomics in Health Care
35
MITIGATING RISKS ASSOCIATED WITH ADMINISTERING
MULTIPLE INTRAVENOUS INFUSIONS: METHODS FOR
ORGANIZING AND ANALYZING PROACTIVE RISK DATA
Andrea Cassano-Piché, M.A.Sc, P.Eng
Human Factors Engineer, Health Technology Safety Research Team
University Health Network
Mark Fan, M.H.Sc
Human Factors Analyst, Health Technology Safety Research Team
University Health Network
Anthony C. Easty, Ph.D, P.Eng, CCE
Health Technology Safety Research Team Leader
University Health Network
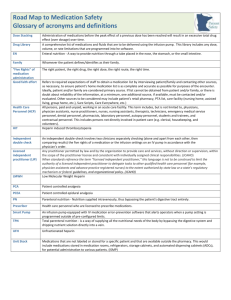
Administering multiple infusions to a single patient is a requisite but risk-prone task that takes place in
many patient care areas. Identifying and prioritizing the associated risks proactively requires detailed
knowledge about the context, including a wide range of medication administration tasks and processes,
which results in a large, highly interconnected data set. This paper discusses the methods used to collect,
organize and analyze risk data related to the administration of multiple infusions in 12 patient care areas
across 10 different hospitals, as well as the patient safety risk themes and associated hospital-based
recommendations identified through this research.
Copyright 2012 Human Factors and Ergonomics Society. All rights reserved. 10.1518/HCS-2012.945289401.006
Introduction A major challenge of collecting and analyzing proactive risk
data using ethnography in the healthcare setting is the volume
and complexity of the data that arises from the number of
degrees of freedom in the system (Savage, 2000). Unlike other
complex socio-technical safety-critical systems where the
physical components and monitoring sensors of the system
being controlled by expert users are fixed, and are defined
primarily by physical principles (e.g., nuclear power plants),
healthcare workers are often required dynamically to construct
the physical systems they control and monitor as they are
caring for patients. This increases the amount of contextspecific data that must be captured and also increases the
difficulty of separating out requisite from extraneous
complexity.
Administering multiple IV infusions to a single patient is a
complex task with considerable patient safety risk (CassanoPiché, Fan, Sabovitch, Masino, & Easty, 2012). It is, however,
a requisite task for many nurses caring for patients receiving
complex treatment and has not been systematically evaluated
in terms of the types of associated risks and the effectiveness
of potential mitigating strategies (Health Technology Safety
Research Team, Institue for Safe Medication Practices
Canada, 2010). It thus warrants a detailed proactive risk
analysis to mitigate potential patient safety risks.
This paper describes a research approach used to
systematically collect and analyze a large, ethnographic field
data set to support a proactive risk assessment of failure
modes associated with administering multiple IV infusions to
a single patient. It also presents the themes of issues identified,
and the corresponding recommendations to hospitals for
mitigating some of the identified issues.
Methods Data Collection Twelve ethnographic field studies were conducted across 10
Ontario hospitals in a wide range of patient care settings
where multiple infusions are frequently administered to a
single patient. Units included critical care environments, an
emergency department, an inpatient oncology setting, and an
outpatient oncology clinic. Four of the 10 field studies were
conducted in pediatric settings and the remaining were
conducted in adult care environments. A mixed methods
approach was followed at each field study, which consisted of
semi-structured interviews and direct observations of nurses
caring for patients receiving multiple IV infusions. Each field
study began with a semi-structured group interview of unit
clinical administrators (e.g., nurse and pharmacy managers),
clinical educators, medication prescribers, and risk managers.
The number and type of participants varied across sites based
on availability. Each interview lasted 2-3 hours and focused
on the unit structure, infusion technologies in use, medication
administration policies and practices, training and education,
and challenges and safety issues related to administering
multiple infusions. The interviews were followed by 2.5 days
of direct observations conducted by two or three human
factors researchers with experience in the healthcare domain.
A nurse consultant on the project also participated in three of
the field studies to further support the human factors
researchers in understanding the clinical context.
36
2012 Symposium on Human Factors and Ergonomics in Health Care
Qualitative field study data were collected in the form of
photographs (Figures 1-2) and written notes. Issues within
each field study were identified and tracked in a spreadsheet.
cause-consequence relationships between issues both within
and across field studies was preserved. The first attempt at
managing the complexity of the data was to group the issues
into task-based themes (Table 1). The themes provided a
useful framework for dividing up the problem space so that
individual researchers could explore each theme
independently and concurrently, but did not make explicit how
contributing factors across the system influenced problems in
multiple themes.
Table 1. Themes of issues identified from the field study data
Themes of Issues
Secondary Infusions
Line Set-up/Removal
Line Identification
Dead Volume Management
IV Bolus Administration
Figure 1. Multiple IV infusions connected to a single IV line
via manifolds
To this end, the data were mapped onto a structural hierarchy
representing the actions and decisions at all levels of the
system (Figure 3). This type of structural hierarchy is a tool of
Rasmussen’s 1997 Proactive Risk Management Framework
(Rasmussen, 2007; Rasmussen & Svedung, 2000). Each
element on the hierarchy (i.e., box) represented a potential
contributing factor to a patient safety issue related to
administering multiple IV infusions. The cause-consequence
relationship between the factors was made explicit by a line
connection creating chains of elements that sequentially led to
outcomes that cause patient harm. Decisions and actions most
directly connected to these outcomes were identified as
primary issues. A risk analysis of the primary issues was
conducted using a Healthcare Failure Modes and Effects
Analysis (De Rosier, Stalhandske, Bagian, & Nudell, 2002).
The HFMEA rating scheme is shown in Table 2. The causeconsequence chains associated with the critical issues were
analyzed to identify potential mitigating strategies.
Results More than 100 field study data elements were mapped onto
the structural hierarchy. Factors with the potential to
contribute to patient harm were identified across all levels of
the system. Twenty-two primary issues were identified, of
which 17 were identified as critical issues based on the
HFMEA risk analysis. Critical issues were defined as primary
issues with a hazard score great than 24.
Figure 2. IV tubing intertwined between the pump and the
patient
Data Analysis The field study data set was large, and presented a challenge
in terms of how to record the data such that the complex
Mitigating strategies were identified for several of the critical
issues, which included at least one mitigating strategy for each
theme except dead volume. Mitigations focused on
interventions that could be implemented at the hospital or
hospital-unit level of the system. The mitigating strategies
were communicated as nine recommendations to hospitals and
are presented in Table 3 and described fully in Cassano-Piché,
Fan, Sabovich et al, 2012.
37
2012 Symposium on Human Factors and Ergonomics in Health Care
Government
109
Lack of usability
standards by
device regulators
(Canada)
Professional
Practice
and
Technology
Regulators
111
Lack of guidance given to
nursing education programs
and hospitals about multiple IV
infusion concepts required in
the curriculum/training program
61
114
105
86
112
Procurement
selection not
optimal for clinical
needs
22
Hospital Budget
constraints
112
49
18
O
r
g
a
n
i
s
a
t
i
o
n
110
Drug library not
configured
optimally
61
86
43
59
M
a
n
a
g
e
m
e
n
t
Setup
Different
pumps used
on different
units
65
87
88
89
90
91
Setup
Pumps need
to be
returned to
home unit
Setup
Lack of
standard drug
concentrations
between units
Setup
Drug library
not configured
for the unit
patient is
transferring to
Setup
Concentration
of drugs is
different
between the
units
Setup
IV tubing/
connectors not
compatible
with new unit
56
22
11
Secondary
Secondary infusions
perceived as low risk
44
Line Diagnosis
No policies or best
practice outlined for
how to exchange IV
setup information
during handover
!"#
38
$%
Secondary
Secondary tubing
connected to the incorrect
port along the tubing (ie
downstream of pump
instead of upstream)
A
4
3
Staff
and
Patient
Activities
Labels
Generic and brand name
used inconsistently across
pump bag and drug tubing
labels for a single patient
(all 3 labels written and
applied by the same RN)
see SRH
Patient is
receiving
multiple
concurrent
IV infusions
$%
98
53
G
Administration
of a
discontinued
medication
Line Diagnosis
Incorrect line
removed
64
Line Diagnosis
Settings changed on
the wrong pump/
channel
Line Diagnosis
Tubing
intertwined like
spaghetti
19
Secondary
Length of time to program via
drug library
$%
30
Line Diagnosis
Incubator and
crib sides
impede the
continuous
tracing of lines
28
Line Diagnosis
Only single
channel pumps
used, requiring
stacking of
pumps
39
Line Diagnosis
Line tracing
process prone
to error
$%
29
Labels
-Illegible
handwriting on
pump/drug tubing
labels
-Poor visibility of
labels
40
41
Labels
Pump/Drug
tubing
labels fall off
Labels
No labels designed
specifically for med
line, drug tubing of
pump labeling in use
42
43
Labels
Syringe labels
applied lengthwise
are obscured by
the syringe clip
Labels
Colour-code scheme
for drug tubing labels
has many similar
colours for different
drugs
49
Labels
Pre-printed, colourcoded drug name
stickers are only
available for some
drugs
Secondary
Secondary
clamp not
unclamped
58
Setup
3-way stopcock
product design
issue
68
Secondary
Bolus of primary
infusion given via
secondary mode
60
Line Diagnosis
Multiple drugs
have same
opaque colour
(propofol, lipids,
breaskmilk, NG
feeds)
65
Secondary
Secondary
medication not
in drug library
Setup
Connecting multiple
stopcocks in series
results in leaks
Pump
Primary infusion
programming error
(wrong values
entered)
100
Dead Volume
CVP line
flushed
99
Labels
External labels
applied to pumps
to provide drug
name and access
info
Dead Volume
One or more rate-critical
medications (fluids) connected
with other (fluids) medications
into a single piece of tubing
79
Pump
Drug library/dose
calculator not used
to program a
primary infusion
66
67
Secondary
No bolus
feature on the
pump
Secondary
Pump software design
prevents the use of the
secondary mode if the
primary line is programmed
using a drug library/drug
dose calculator
(Graseby, Baxter...?)
Labels
Drug libraries do
not display drug
name
prominently or
indicate access
information
105
98
80
75
Secondary
No bolus
appropriate
profiles in the
drug library
54
78
Labels
External pump labels
not removed prior to
setting up a new
infusion on the pump
9
Dead Volume
Rate-critical
medication
administered via
CVP line
Pump
Highly potent
medications
administered
concurrently via a
pump with poor flow
rate accuracy
74
Setup
Use of recalled 3-way
large bore stopcock to
connect multiple IV lines
55
Pump
Single interface used
to program multiple
pump channels/
modules
69
Secondary
Secondary infusion
programming error
59
!"#
26
Line Diagnosis
IV hooks
arranged
circularly on IV
poles
102
Setup
Pump label-bag
mismatch (label applied
after bag attached +
pump programmed)
83
A
73
$%
D
Required
medications
not delivered
to the patient
31
Line Diagnosis
Transporting/
ambulating
patients
tangles the
lines
Setup
Nurses try to space the IV bags
apart to make the bag labels
more visible
Setup
Multiple IV infusions connected
at the same time
51
!"#
Secondary
Back flow of
secondary infusion
into primary infusion
Secondary
No drug library available for
secondary infusions (SMH/
Colleague)
Setup
Colleague pumps tubing needs
to be moved up over time
103
A
Infusion delivered at
incorrect rate
47
18
Secondary
No backcheck valve
on primary tubing
Line Diagnosis
Slower running line
attached upstream of
faster running line(s)
and no chaser/driver
attached
E
48
10
113
104
Setup
Patient
transfers from
one unit to
another (or
from EMS)
101
108
Line Diagnosis
Line tracing
prcess lengthy
$%
Labels
Drug tubing labels
wrapped around IV
tubing leave part of
the drug name
obscured
Setup
Batch process of steps during
setup of multiple IV infusions
Setup
IV bag-pump mismatch (pump
programmed before bag
connected)
High risk
medication
delivered
!"#
$%
Labels
Inconsistent use of
colour to identify CVC
lines across vendors
Setup
Sequential
intermittent infusions
hung in advance of
being connected to
the pump
93
Patient
Harm
17
16
84
!"#
32
IV medications
not D/C when
other
administration
routes become
available
27
Labels
Med lines
not labeled
(pump)
70
!"#
24
Secondary
Secondary IV
bag connected
to incorrect
primary line
25
Line Diagnosis
IV Bags do not
physically align
with the pumps
they are
attached to
Equipment
and
surroundings
E
Delay/
interruption in
administration
of critical
medications
63
Dead Volume
Medications in dead volume of new
tubing not in correct proportions for
undermined length of time
!"#
92
Setup
Wrong IV bag
connected to
the pump
76
94
52
Line
Diagnosis
Line identified
incorrectly
106
Secondary
Bag height
difference
not great enough
15
Secondary
Secondary infusion
not programmed via
the drug library
Setup
RN height/
reach
Setup
IV bag-programming mismatch
(pump programmed after bag
connected)
F
Bolus of
incorrect fluid/
medication
administered
51
Setup
Limited
central IV
access
14
$%
C
Vessicant medication
in PIV
34
Secondary
Some hangers not
long enough for
longer IV bags
Labels
No drug
tubing labels
applied to
lines
62
Setup
Secondary hangers used to
lower primary bags when no
secondary infusion is running
77
Dead Volume
Medication unhooked
from 2 or 3-way ystyle connector and
there residual volume
in the line is not
identifyable
13
5
Line Diagnosis
Medication connected
to/disconnect from
incorrect line
$%
Labels
Med lines not
labeled (tubing)
99
68
35
!"#
Setup
IVs need to be
rearranged prior to
patients going for CT
imaging (ie free up a
PIV line)
RNs work at
more than one
organization
18
23
$%
6
1
14
85
57
Line Diagnosis
Secondary medication
injected into incorrect
line (tubing or buretrol)
(pediatrics)
45
B
Incompatible
medications
running together
23
Inadequate training on
managing multiple IV infusions
12
$%
Setup
Backflow can
occur through Y
connectors for 2
primary lines if
pressure
differential too high
Labels
Patient access
information on
the pump label
is inaccurate
$%
8
Labels
Label placed
on incorrect
drug tubing line
50
Line Diagnosis
Line setup information
not formally
communicated at
handover
12
7
107
11
$%
37
Line Diagnosis
No standardized
approach to line
setups
(arrangement,
components
used, etc)
!"#
Dead Volume
No standard process for
managing the dead
volume of a 2 or 3-way ystyle connector if a drug is
discontinued and removed
!"#
21
Dead Volume
No standard means of
communicating that a fluid
should not be bolused
because it is connected to
other medications with
sensitive infusion rates
36
Secondary
Unnecessary port
on primary tubing
just below the
pump
!"#
Secondary
Lack of experience
setting up
secondary
infusions
$%
Labels
Inconsistent use of
terminology for drug
abbreviations and
access locations on
labels (between RNs
on same unit)
$%
!"#
$%
9
106
81
114
35
Dead Volume
Significant change in rate of one
or more of the fluids connected
to other rate-critical medications
Dead Volume
IV connector design
results in dead
volume
96
Line change
required
Setup
Tubing too short to
reach between bag
and pumps when
multiple pumps are
stacked vertically
97
Setup
Date of IV
line (based
on policy)
Secondary
Traditional
pump used
61
Pump
Software/User
interface
Design issues
Figure 3. Structural Hierarchy of Factors Contributing the Multiple IV Infusion Administration Risks
Table 2. Healthcare Failure Modes and Effects Analysis
Rating Scheme for Multiple IV Infusion Issues
Rating
1
Detectability
No checking process is required to help detect the error.
2
A checking process is required to help detect the error,
but this process is not well defined and relies on human
vigilance.
3
The error is not detectable based on current standards for
knowledge/experience.
4
Rating
1
2
The error cannot be detected by any reasonable human
process.
Likelihood
Remote = the error may happen within the next 5 years
Occasional = the error is likely to happen within the next
1–2 years
4
Frequent = the error is likely to happen within the next
year
Severity
Minor = Error results in no harm, or the potential harm is
unknown.
Moderate = Patient is temporarily harmed by the error.
Severe = Patient is permanently harmed by the error.
Critical = The error causes the patient’s death.
2
3
4
1
2
3
Uncommon = the error is likely to happen within the next
2–5 years
3
Rating
1
Table 3. Recommendations to hospitals based on an analysis
of the structural hierarchy
Recommendation
4
5
6
When initiating a secondary medication infusion (often
referred to as a “piggyback” infusion), nurses should verify
that the secondary infusion is active, and that the primary
infusion is not active, by viewing the activity in both drip
chambers. Full drip chambers should be partially emptied to
restore the visibility of drips.
Continuous high-alert medications (Institute for Safe
Medication Practices Canada, 2005) should be administered as
primary infusions. Continuous high-alert medications should
not be administered as secondary infusions.
Secondary infusions should be attached to primary infusion
sets that have a back check valve. If infusion sets without back
check valves are also available on the unit, multiple strategies
should be employed to ensure that the types of tubing available
are easily differentiated, and that the likelihood of a mix-up is
minimized.
Hospitals should work towards the use of gowns that have
snaps, ties, or Velcro on the shoulders and sleeves.
If an “emergency medication line” that is controlled by an
infusion pump is set up on a patient, it is strongly suggested
that the associated primary IV tubing be labelled as the
emergency medication line at the injection port closest to the
patient. The label should be prominent, and visually distinct
from all other labels in the environment.
When setting up multiple IV infusions at the same time (e.g., a
new patient requires many ordered infusions immediately,
routine line changes), infusions should be set up one at a time,
as completely as possible, before setting up the next infusion.
Set-up tasks required for each infusion vary and may include:
•
labelling (e.g., IV tubing, pump);
•
spiking and hanging the IV bag;
•
connecting the IV tubing to the pump;
•
programming the IV pump;
38
2012 Symposium on Human Factors and Ergonomics in Health Care
connecting the IV tubing to the appropriate
location (e.g., patient access, manifold); and
•
starting the pump (unless a secondary infusion
must be set up prior to starting the pump, or
other infusions need to be connected to a multiport connector before flushing).
Minor modifications to this recommendation are required for
routine line changes.
Multiple 3-way stopcocks joined together in series to connect
multiple IV infusions into a single line are prone to leaks,
which may often be undetectable. Hospitals should provide
multi-port or multi-lead connectors, and nurses should use
these connectors to join multiple IV infusions into a single
line, as required.
Hospitals should develop a policy to limit the practice of
manually increasing the infusion rate to administer a
medication bolus of a primary continuous infusion. If a
medication bolus is to be administered using the primary
continuous infusion pump/pump channel, then the nurse should
program the bolus dose parameters (i.e., total amount of
medication to be given over a defined duration) into the pump
without changing any of the primary infusion parameters.
Some examples may include the following:
•
programming a bolus using a dedicated bolus feature
in the pump
•
programming a bolus using the pump’s secondary
feature
Hospitals should ensure that their smart pump drug libraries
include hard upper limits for as many high-alert medications as
are appropriate for each clinical area, in order to prevent the
administration of a bolus by manually increasing the primary
flow rate.
organizations can each identify their own unique subset of issues from the set of factors presented on the
hierarchy to create their own individual structural
hierarchy as a first step towards developing
mitigating strategies most important to their
organization.
•
7
8
9
The nine recommendations identified in this study focused on
the lower levels of the hierarchy, where human factors
recommendations are traditionally made, because these
recommendations can be immediately implemented to
minimize risks. Mitigating strategies aimed at higher levels of
the system (e.g., changes to clinical education programs, new
standards for infusion systems) and solution approaches that
require further study prior to determining the best mitigation
approach are currently being investigated in the next phase of
the study.
References 1.
2.
3.
Discussion The human factors proactive risk analysis of the processes
associated with administering multiple IV infusions revealed
that multiple IV infusion administration is a complex, riskprone process that requires a systems approach to risk
mitigation. The structural hierarchy tool from Rasmussen’s
risk management framework (Rasmussen & Svedung, 2000)
proved useful for organizing complex healthcare field study
data, such that the relationship between many factors that
influence human performance across levels of the system was
explicit. This has several important implications for how riskmitigating strategies are developed.
1.
2.
3.
It supports a systematic analysis of the effect of a
potential risk-mitigating strategy on all other
elements of the system by making the relationship
between all the elements explicit. This helps to
minimize unintended negative consequences
associated with changes to the system.
It supports a focus on solutions at higher levels of the
system (e.g., government, regulatory bodies) than are
usually considered, because the structural hierarchy
tool prompts an inclusion of contributing factors at
these levels.
It facilitates the aggregation of information across
field sites, making the analysis more generalizable
across the healthcare domain. Individual healthcare
4.
5.
6.
7.
Cassano-Piché, A., Fan, M., Sabovich, S., Masino, C., &
Easty, A. C. (In Press). Multiple Intravenous Infusions
Phase 1b: Practice and training scan. Ont Health Technol
Assess Ser [Internet] . Toronto, ON, Canada
De Rosier, J., Stalhandske, E., Bagian, J. P., & Nudell, T.
(2002). Using Health Care Failure Mode and Effects
Analysis: The VA National Centre for Patient Safety's
prospective risk analysis sistem. Joint Commission
Journal of Quality Improvement , 28 (5), 248-267.
Health Technology Safety Research Team, Institue for
Safe Medication Practices Canada. (2010, 09 24).
Multiple Intravenous Infusions Phase 1a: Situation Scan
Summary Report. Retrieved 03 19, 2012, from Health
Technology Safety Research Team Web site:
http://www.ehealthinnovation.org/files/Multiple%20IV%
20Infusions_Phase1a_SummaryReport.pdf.
Institute for Safe Medication Practices Canada. (2005, 02
1). Secondary Infusions Require "Primary" Attention.
ISMP Canada Safety Bulletin , 5 (2), pp. 1-2. Available
at: http://ismpcanada.org/download/safetyBulletins/ISMPCSB200502SecondaryInfusions.pdf.
Rasmussen, J. (2007). Risk management in a dynamic
society: A modeling problem. Safety Science , 27, 183213.
Rasmussen, J., & Svedung, I. (2000). Proactive risk
management in a dynamic society. Karlstad, Sweden:
Swedish Rescue Services Agency.
Savage, J. (2000). Ethnography and health care. BMJ ,
321, 1400-1402.
Acknowledgements This research was commissioned by the Ontario Health Technology
Advisory Committee, funded by Health Quality Ontario and
conducted in collaboration with the Institute for Safe Medication
Practices (ISMP) Canada. The authors wish to thank the members of
the Multiple IV Infusions Expert Panel, the AAMI Multiple Line
Management Working Group and the health care staff who supported
and participated in the field studies.