Chemistry 110 B Syllabus
advertisement

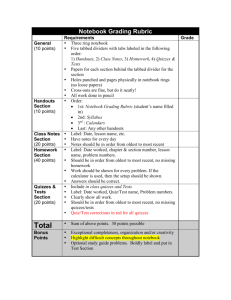
Chemistry 110 - 02A (4782) Chemical Concepts Winter 2016 Syllabus Class Day: Time: Location: Monday, Tuesday, Wednesday, & Thursday 8:30 – 9:20 am Building 15 Room 302 Lab Day: Time: Location: Friday 7:30 – 9:20 am Building 15 Room 324L Instructor Office: Telephone: e-mail: website: Philip Hunter Building 15, Room 338 253.566.5302 phunter@tacomacc.edu (Begin the subject of all messages with "Chem 110".) www.tacomacc.edu/home/phunter Office Hours 9:30 – 10:20 am Monday, Wednesday, and Thursday in my office (15-338) 9:30 – 10:20 am Tuesday and Friday in the Active Learning Lab (aka the Fish Bowl) I welcome you to see me during my office hours. You may also make an appointment to see me at other times, or just stop by when my office door is open. Class Web Sites Chemistry 110 B webpage (www.tacomacc.edu/home/phunter/chem110b.shtm) contains links to the syllabus, homework, handouts, and class notes. Grades and Panopto lecture captures are available through Canvas on the Portal. Prerequisite Math 95 or higher, or TMath 100. These may be taken concurrently with Chemistry 110. Supplies Required items are Chemistry for Changing Times, 14th ed., Hill and McCreary (ISBN 9780321972026 or 9781323228265). Discovering Chemistry: A Laboratory Manual, Hunter and Orchard. A lab notebook bound with thread. I suggest a composition book. Three ring, spiral, and perforated notebooks are not acceptable. See the Lab Notebook section below for more details. A black or dark blue permanent ink pen for writing in the lab notebook. A calculator. A basic scientific calculator such as the TI-30 is recommended. Optional item is: Chemical splash goggles with indirect vents. These must be approved by me. Course Description This course is a survey of the fundamental principles of chemistry. It includes the study of the metric system, atomic theory, bonding, properties of matter, reactions, nomenclature, and applications of chemistry to everyday life. This section also includes the study of nuclear chemistry. It includes laboratory work. The course is for students who have not previously completed a course in chemistry. (5 credits) Academic Assistance I am one of many resources available to help you succeed in this class. Here are some other resources. Study Groups You should join or form a small study group for this class. The vast majority of students find that active participation in a study group improves their performance in their classes. Active Learning Lab The Active Learning Lab on the first floor of the Science & Engineering Building is a great place to study. Chemistry faculty and tutors are often available in the room, as are other chemistry students. Writing and Tutoring Center Chemistry tutors are available at no additional cost in the Writing and Tutoring Center on the second floor of Building 7. These tutors may also be available in the Active Learning Lab. The Writing and Tutoring Center also offers one-on-one help with your writing projects, including help organizing a paper, adding details, improving grammar, polishing the draft, or documenting sources. Call 253.566.5184 for an appointment. For more information see www.tacomacc.edu/wtc. Counseling & Advising Center The Counseling & Advising Center staff in Building 7 can help you address personal difficulties that interfere with your studies. This includes things like test anxiety. Call 253.566.5122. For more information see www.tacomacc.edu/resourcesandservices/counseling. Disability Accommodations All students are responsible for all requirements of the class, but the way they meet these requirements may vary. If you need specific auxiliary aids or services due to a disability, please contact the Access Services office in Building 7 (253.566.5328). They will require you to present formal, written documentation of your disability from an appropriate professional. When this step has been completed, arrangements will be made for you to receive reasonable auxiliary aids or services. Access Services will prepare a disability accommodation document. You must give me this document. Because this is a confidential matter that requires discussion between us regarding how to provide the aids or services, you must give me the document at a meeting in my office. This must be done before the accommodation is needed so that appropriate arrangements can be made in advance. Degree Learning Outcomes Tacoma Community College has identified six degree related learning outcomes for all students who complete a degree at the college. These outcomes are available at www.tacomacc.edu/abouttcc/missionvisionandstrategicplan/ Page 2 of 13 Natural Science Student Learning Outcomes Tacoma Community College has identified five learning outcomes for Natural Science Distribution Courses. These outcomes are available at http://www.tacomacc.edu/catalog/1516catalog/program-learning-outcomes-plo.htm. Course Learning Outcomes Upon satisfactory completion of the course, students will be able to: 1. Work safely in the laboratory and demonstrate the basics of safe chemical use and disposal. (PLO: 4,5) 2. Collect and analyze experimental data. (PLO: 4,5) 3. Work as a member of a team to perform chemistry experiments and to present the results. (PLO: 4,5) 4. Relate classroom and laboratory experiences to phenomena outside the classroom. (PLO: 1,5) 5. Describe the process of science. (PLO: 2) 6. Use the metric system in measurements and calculations. (PLO: 5) 7. Explain the atomic nature of matter. (PLO: 3) 8. Describe the basic atomic structure, including subatomic particles. (PLO: 3) 9. Use the periodic table to predict atomic properties and trends. (PLO: 3) 10. Explain how atoms form ionic and covalent bonds. (PLO: 3) 11. Name simple compounds from formulas and write formulas from compound names. 12. Write and interpret chemical equations and perform stoichiometric calculations. 13. Explain macroscopic behaviors of substances using the properties of atoms and molecules. (PLO: 3) 14. Define oxidation and reduction in terms of a transfer of electrons and recognize simple redox reactions. 15. Identify acids and bases and use pH to characterize solutions. 16. Recognize a few classes of organic compounds and explain the role of functional groups in their chemical and physical properties. 17. Recognize the major classes of compounds found in living organisms and explain their structures and functions. 18. Communicate your understanding of chemistry using the chemical vocabulary. 19. Apply critical thinking to chemistry problems. Attendance Regular attendance is necessary if you are to succeed in Chemistry 110. You should attend every class period. If you miss a class, you are responsible for finding out what you missed and learning the missed material before the next class session. Students who habitually miss class are seldom successful in this course. Attendance in lab is required and roll is taken. See the Laboratory section for the policy on missed labs. Instructional Methods In this class we will use a variety of instructional methods including lectures, demonstrations, labs, and team work. Each of these methods has its advantages and disadvantages. You are probably more familiar, and comfortable, with some methods than with others. However, I expect you to participate in all activities and to approach the activities in a positive, professional manner. Page 3 of 13 Classroom Etiquette Students come to class to learn. It is expected that everyone will act in ways that are respectful and that promote learning for all students and will refrain from actions that interfere with learning. Expectations include Arrive on time. If you arrive late, do your best to minimize the disruption to other students. If I will be late, I will send someone to inform you. Use the time to study. Stay until the end of class. If you must leave early, sit near the exit and minimize the disruption to other students. Come ready to learn. Even quiet conversation is very disruptive during class. Except during team activities, you should not talk to other students unless you have the floor. If you cannot hear what is said or see what is written, tell me immediately. Learn the names of the people you interact with in class and lab. Most students call me "Mr. Hunter". Guests, including children, are welcome in class on an occasional basis with my prior approval. Guests must not disrupt the class in any way. You are responsible for your guests. Guests are not allowed in the laboratory. Turn off all alarms and ringers before class starts. Beverages are allowed in the classroom. Food should generally not be eaten in class, except as necessary to maintain your health. Food and beverages are not permitted in the laboratory. If you have the flu, stay home. I can usually answer a few quick questions before and after class. However, lengthy discussions, and confidential or private discussions should take place in my office. Laboratory The laboratory is an integral part of Chemistry 110. It is included so you can experience chemistry. You are expected to actively participate in all the experiments. Before coming to the lab, study the experiment so that you are familiar with it. The assigned experiments are listed in the calendar at the end of this document. Learn the names and formulas of any new compounds. Prepare your lab notebook as described in the Lab Notebook section below. All students must follow the chemistry lab safety procedures and standard operating procedures established by Tacoma Community College, the Science & Engineering Department, and the instructor. Students who repeatedly or willfully violate these procedures may face sanctions, including removal from the course, a failing grade, and referral to the college for action under the Code of Student Rights and Responsibilities. The departmental safety procedures for chemistry are available at http://cms.tacomacc.edu/UserFiles/Servers/Server_6/IntranetFile/Chem.%20Lab%20Safety,% 20Procedures,%20Emergencies2.pdf. All Chemistry 110 students must pass a Chemistry Lab Safety Quiz to remain enrolled in the class. Missed Labs. If you miss a lab due to illness or other event beyond your control, you are responsible for meeting with me to arrange for a make-up assignment. If possible, contact me before the missed lab begins. Other missed labs may not be made up. Page 4 of 13 Lab Notebook Although there is no standard format for all science lab notebooks, there are elements that are expected in any good laboratory notebook. These include the following. a. The notebook must be thread-bound. Spiral, loose-leaf, perforated, and other notebooks where pages may be easily removed or added are not acceptable. b. Never tear pages out of your notebook or add pages to it. c. Observations and data must be immediately recorded directly into the notebook. Do not write them on other paper or keep values in your head. Do not delay writing them down. Take the notebook with you if you are going to make any observations. d. Black or blue permanent ink must be used to record all information in your notebook. Felt tipped pens are usually a bad choice since the ink will run if water or organic solvent is spilled on the notebook. Pencil or erasable ink should never be used. e. If you make a mistake, cross it out with a single line. You should still be able to read the original entry. Like this. Not like this: . And not overwritten like this: . Record the correct entry nearby. f. Measurements include units. g. The first two or three pages should be used for a Table of Contents. This should be updated as experiments are added to the notebook. The Table of Contents should include the subject, page number, and date of each entry. h. All pages should be numbered. Often you must number the pages yourself. i. Every page in the notebook should include the date the entries were written. j. Do not skip pages, except for the two or three pages left at the beginning for the Table of Contents. If a page or large part of a page is left blank, cross it out with one diagonal line and add a note like “Page Left Blank” and the date. k. Begin each lab on a new page. l. Many laboratory notebooks also include a Table of Abbreviations. It is not necessary to include standard symbols or abbreviations in this table. However, if you develop your own abbreviations or use abbreviations that are not widely known, you must have a Table of Abbreviations. It may be immediately after the Table of Contents, or in the back of the notebook. You should keep complete records of your experiments in your lab notebook following the guidelines above. Lab Reports Lab reports will be individual reports in which you will write responses to questions regarding what you observed in the lab. These reports are worth 25 points each. For each lab report, you should also do some library research on a narrow topic related to the experiment and tell what you learned from this research. Do not simply copy sentences or paragraphs from the web into your paper, as this is not research. You should follow the standards of good English writing. Make frequent use of your writer’s reference book. Due dates for lab reports are given in the Calendar at the end of this document. Lab reports are due by 2:00 pm. Page 5 of 13 Graphing Guidelines The guidelines below are adapted from the TCC Mathematics Department Student Graphing Guidelines. You are expected to follow the graphing guidelines below on homework, lab reports, and test questions that require an accurate graph. AXES: Axes and any straight lines are drawn with a straight edge. The scale is clearly indicated on each axis. Each axis is labeled with the meaning and units of the axis. ACCURACY: Graph paper, engineering paper, or a computer is used. The scale is chosen so that values between the tick marks or grid lines can be easily interpolated. Scales are usually linear. Logarithmic scales may be used if appropriate. Other scales are seldom, if ever, used. On spreadsheet programs “x-y” or Physical Properties of Copper 70 “scatter” charts are usually used; “line” charts should be avoided. 60 m = 8.87 V + 0.31 Mass (g) CLARITY: 50 If multiple functions are graphed on a single set of axes, each is 40 Data identified in a legend. Fit Line 30 Unrelated problems are graphed on separate axes. 20 The size of the graph is helpful: it 2 3 4 5 6 is neat, big, and dark enough to be Volume (ml) easily read and understood. A graph should usually occupy between a half and a whole sheet of paper. The scale and origin are chosen so that the data and lines use a large fraction of the graph. SYMBOLS & ANALYSIS: Measured data are indicated with symbols such as circles, triangles or squares. The trend is shown as a line. This line usually shows the overall pattern in the data and does not simply connect the data points with straight or curved segments. Studying Outside of Class Regular study is important to succeed in this class. You should plan to study at least 12 hours each week outside of class. It is best to study every day. Use this time to review your notes, study the text, work and rework homework problems, prepare for lab, write lab reports, and rework quiz questions. You should begin working on an assignment as soon as it is assigned. You should find and use the study methods that are most efficient for you. Page 6 of 13 7 Homework Homework is assigned throughout the quarter. It is essential that you do the homework. Much of the learning occurs as you struggle with the homework. Begin working on homework questions as soon as you receive the assignment and work on it daily. The order of questions on the homework is typically the same as the order we will study the topics in class. To become accomplished at any skill requires practice and repetition. This is true whether it is playing a musical instrument, playing a sport, drawing, or reading. The same is true for learning chemistry (or math or many other subjects). Solving homework problems is one way to practice. Solving a homework problem once is not sufficient, just as making one free throw is not sufficient to be a good basketball player. You should frequently rework homework problems you have answered previously, especially difficult ones. If you struggle to solve a homework problem, then as soon as you have finished solving it, do the same problem again. Do the problem again just before you go to sleep. Do it again the next morning. Make sure you can do that problem under exam conditions. Keep coming back to the same problem frequently enough that you can do it with ease under exam conditions. Every field has material you must memorize. Most homework assignments will include some memorization. I strongly recommend you make and use flashcards. I cut 3 × 5 cards into quarters for this. Carry them with you and review them frequently: walking between classes, waiting in line, eating breakfast. The most important time to review them is just before you go to sleep. Submitted Work All work must follow these guidelines. Use standard English. Consult your writer’s reference for details. For chemical words and symbols, use the standards present in the course text. Use only black or dark blue ink, or pencil. Only ink may be used in the lab notebook. Use only white paper. The paper may be ruled, grid, or blank. Graphs must be on graph paper or be computer generated. See Graphing Guidelines for more details. Work of more than one page must be stapled in the upper left corner. Work that is hidden behind a staple will not be graded. Do not place work in any type of binder or cover. Include "Chem 110 - 02", the assignment title, your name, and my name in the top right corner of the first page. All typed work should also follow these guidelines. Use a standard 12 point font (i.e. Arial, Garamond, Times New Roman, or Verdana) Double space. 1 inch margins on all sides. You may submit work these ways. Hand it to me. Give it to the secretary in Room 102 of the Science & Engineering Building. Make sure my name is on the first page. Page 7 of 13 Citations You must properly cite any sources you use to complete your lab reports or homework. Citations may be in any widely used style. See your writer’s reference, or the Citing Sources library page on MyTCC (you must log in first) for more information about proper citations. Facts from Hill and McCreary or class that you use to answer homework questions do not require a citation. Answers copied from any source and all direct quotes from any source always require proper citation. Quizzes and Exams Quiz dates are listed on the Course Calendar. The lowest two quiz scores are dropped and do not count toward the final course grade. There are no make-up quizzes. Missed quizzes are assigned grades of zero and are eligible to be among the two dropped quizzes. (If a quiz receives a grade penalty due to academic dishonesty it will be included in the final course grade. Such a quiz will not be included when determining which quiz scores are dropped.) Quizzes may include questions about the laboratory. Quizzes may be cumulative. The scope of each quiz will be given on the homework assignment sheet or announced in class. A two hour final exam is given. The exam may include questions about the laboratory. The exam is cumulative. Only exams missed due to dire emergency may be made up or taken early. I must be notified before the missed exam if possible. Travel is not a dire emergency. Do not schedule travel on exam dates. The exam date is listed on the Course Calendar. Make sure your family and friends know you cannot travel on this day. If you need to look in your text or notes to answer the homework problems you are not prepared for the quiz or exam. When you study for a quiz, you should write one for yourself. Then take your quiz under exam conditions – time limit, no texts or notes, etc. The following may not be used in any way during exams and quizzes: Calculators with the capability of wireless communications. Calculators with chemistry or science memory packs. Dictionaries, including paper and electronic translation dictionaries. Cell phones, mp3 players, or any other electronic device other than a calculator. A calculator app. You may not share calculators during exams or quizzes. Calculators A calculator is necessary for the final exam, and some homework, labs, and quizzes. Wireless Communication Devices Wireless communication devices, such as cell phones, may not be used in any way during exams and quizzes. In case of a bona fide emergency, you can be contacted through the security office (253.566.5111). A ringing cell phone or talking on a cell phone in class is extremely disruptive to learning and is unacceptable. Communication devices must be silent in the classroom and labs. A ringing cell phone or similar event during class or lab may result in a penalty for the user. A student who talks on a cell phone in class will be asked to leave and will be referred to Student Services for disciplinary action. Page 8 of 13 Student Suggestions and Concerns I welcome your ideas on ways to improve learning in this class. If you have concerns about this class, please meet with me about them in my office to discuss them. The classroom is usually not an appropriate venue for such discussions. If we are unable to resolve your concerns, you may wish to meet with Katie Gulliford, the Science & Engineering Department Chair. You should see her in her office in Building 15 Room 335. Academic Integrity The reason to study chemistry is to learn. As stated in the TCC catalog, “Students are expected to be honest and forthright in their academic endeavors. Cheating, plagiarism, fabrication or other forms of academic dishonesty corrupt the learning process and threaten the educational environment for all students.” The following examples are written to clarify the expected standards in this class. If a standard is unclear to you, you should ask me for clarification. Exams and quizzes are solely your work at the time of the examination. Circumventing or attempting to circumvent the restrictions placed on an examination is a form of cheating. Examples of cheating include obtaining or supplying answers, obtaining or supplying questions on an exam or quiz prior to its administration, using unauthorized notes during the exam or quiz, looking at another student's work, placing your work where it can be more easily seen by another student, sharing calculators, or using wireless communication devices. The appearance of cheating should also be avoided. For example, notes should not be placed in your calculator case, or another place where they may be viewed during an exam or quiz. I encourage you to work together on homework, lab problems, reports, and presentations. When you work together, help each other understand the material. However, what you turn in must be in your own words, the product of your own work and research, and represent your understanding of the material. Presenting another person's words, work, data, ideas, or answers as if they were your own is plagiarism. Even if another person gives you permission to use her work, you must give that person proper credit. Data, words, ideas, and information taken from other sources must be properly cited. This includes information from the Internet. If you use another person's data, results, or ideas, clearly state that you have done so and give the person credit by including a complete citation. This includes answers copied from the text. If you copy another person's words, you must place all the text involved in quotes or indent it, and you must give a complete citation. You must clearly show what text you have paraphrased and give a complete citation. If you are unsure about when or how to give a complete citation, request clarification before you submit your work. (Avoiding Plagiarism on MyTCC may help, or simply ask your instructor.) If you work with other students on a homework problem to such an extent that your answers are essentially the same, state this at the end of the solution, including the names of the students. This should occur on only the most challenging problems. Inventing or modifying laboratory data is fabrication. If you believe your data are in error, state why, and how you have analyzed the data. If you use another person’s data in a lab report, clearly state whose data you are using. Page 9 of 13 Coursework that receives a grade penalty due to academic dishonesty will be included in course grading. The consequences of academic dishonesty may range from a verbal warning to expulsion from the College in accordance with the Code of Student Rights and Responsibilities. For example, work containing plagiarized or fabricated information will be rejected and a grade penalty will be imposed. A student who cheats on a quiz or exam will receive a zero on the assignment. A student involved in a significant violation of academic integrity may receive a failing grade for the class. The College’s Code of Student Rights and Responsibilities is available in the office of the President of the Associated Students, the Library, and the Associate Vice President for Student Services’ office. The complete Administrative Procedure for Academic Dishonesty, which includes definitions of the forms of academic dishonesty and the appeals process, is available on the TCC website at www.tacomacc.edu/cms/One.aspx?portalId=43428&pageId=54163. Withdrawals and Incompletes You may withdraw from the course without receiving a grade through January 15. You may withdraw through February 26 and receive a W grade. You must withdraw through the Enrollment Services Office in Building 7. A withdrawal after this date (WI) will be granted only in an extreme case involving circumstances beyond your control. If you must withdraw for medical reasons, immediately consult with the Enrollment Services Office regarding a medical withdrawal. If you might be activated for military service during the quarter, contact the Enrollment Services Office in advance for procedures for this case. An incomplete will be granted only in an extreme case involving circumstances beyond your control. You must have completed almost all the course work. The college requires that an Incomplete Contract be signed before you can receive an incomplete. If you are enrolled in this class and do not officially withdraw, you will receive a grade based on the points you earn. See the Grading section below for more details. Grading Grade A B C D E Description Honor Good Average Minimum passing grade Failure to complete minimum requirement Grading will NOT be on a curve. You will be graded according to how well you demonstrate your understanding of the material taught in this course. This means that you are not competing against each other for a few A's. Instead, you should help each other learn the material. You are each other's best help. Page 10 of 13 The final grade will be determined as follows. Homework: 11 @ 10 points each 110 points Final Exam 200 Quizzes 6 @ 50 points each 300 Lab Reports 8 @ 25 points each 200 Lab Notebook 20 Note: The number of assignments may change. This will result in a change in the total points. The points per assignment will stay the same. The course grade will be determined from the percentage of the possible points earned using the table below. As described in the Quizzes and Exams section, the lowest two quiz scores are not included in the course grading. You may calculate your current grade at any time by calculating your percentage earned: (total points you have earned) ∕ (total points possible so far) × 100% A 93 – 100% A- 90 - 93% B+ 87 - 90% B 83 - 87% B- 80 - 83% C+ 77 - 80% C 71 - 77% C- 70 - 71% D+ 67 - 70% D 60 - 67% E <60% Students who do not complete the laboratory assignments will be have their grade adjusted as follows: # of Labs assignments missed Highest Possible Course Grade 2 B 3 C 4 D 5 E Coursework that receives a grade penalty due to academic dishonesty will be included in course grading. Homework Grading Scheme Neat, orderly, complete, and correct homework will receive 10 points. Incomplete or incorrect homework will receive points approximately proportional to the amount that is completed correctly. Homework must follow the guidelines in the Submitted Work section above. Homework that is difficult to grade due to disorganization or illegibility will not receive full credit. Homework is due by 8:30 am. Because homework solutions will be provided, late homework is not accepted if it is 24 hours or more late. Lab Notebook Grading Scheme The grading scheme for the lab notebook is based on adherence to the guidelines given above in the Lab Notebook section. 14 points for following Guidelines a – d. 2 points for following Guideline e. 3 points for following Guidelines f – h. 1 point for following Guidelines i – l. The lab notebook is due by the beginning of the final exam. Page 11 of 13 Late Work Homework is not accepted beyond 24 hours late since solutions will be provided. Work submitted up to 24 hours late will receive 70% credit. Work submitted between one and five days late will receive half credit. Work more than five days (120 hours) late will not be accepted. Work will not be accepted after 3:00 pm, Tuesday, March 15. This syllabus and calendar are subject to change. Any changes will be announced in class. It is probably needless to recommend the subject: chemistry is recognized as a science of such general interest, such wide usefulness, and such universal application, that no intelligent person can endure long to remain ignorant of its principal facts and laws. Preface to Chemistry by John Howard Appleton, 1884 Page 12 of 13 Chemistry 110 - 02 Winter 2016 Tentative Course Calendar Monday Tuesday Wednesday Thursday Friday January 4 5 6 7 HW 1 Due 8:30 am 8 Lab Safety & Lab A part I 11 Lab Safety Quiz 12 HW 2 Due 13 14 Quiz 1 15 Lab B Lab A part I Report Due 18 MLK Holiday College Closed 19 HW 3 Due 20 21 Quiz 2 22 Lab C Lab B Report Due 25 26 HW 4 Due 27 28 Quiz 3 29 Molecular Models Lab February 1 2 HW 5 Due 3 4 Educational Planning Day No Day Classes See your adviser 8 9 HW 6 Due 10 11 Quiz 4 5 Meet in 15-302 7:30 – 9:20 Lab C Report and Molecular Models Report Due 12 Lab J 15 President’s Day Holiday College Closed 22 16 HW 7 Due 17 18 Quiz 5 19 Lab H Lab J Report Due 23 HW 8 Due 24 25 Quiz 6 26 Lab F Part I Lab H Report Due 29 March 1 HW 9 Due 2 3 Quiz 7 7 8 HW 10 due 9 10 Quiz 8 4 Lab E Lab F Part I Report Due 11 HW 11 Due 7:30 am Meet in 15-302 7:30 – 9:20 Lab E Report Due 14 15 Final Exam: 8:30 am – 10:30 pm Lab Notebook Due: 8:30 am Last Chance to turn in work: 3:00 pm Page 13 of 13