Investigating the Properties of Enzymes
advertisement

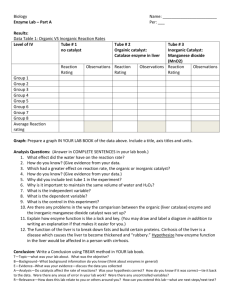
The Bronx High School of Science Valerie Reidy, Principal Department of Biology J. Donahue, IA Asst. Principal Name Date ACTIVITY 16: Investigating the properties of enzymes (Reminder: Bring goggles for this lab) (full lab write-up) Introduction: It is the presence of molecules called enzymes that give cells the ability to carry out the chemical reactions necessary for life. Enzymes have the following characteristics: 1) They are organic catalysts. A catalyst is a chemical agent (inorganic or organic) that changes the rate of a reaction. In the case of enzymes, they usually speed up reactions. 2) They participate in the reaction, but remain unchanged in the end. (Common to all catalysts) 3) They are protein in chemical composition. 4) All formal names of enzymes end with ase. Many cells produce hydrogen peroxide (H2O2) as a by-product of their metabolism. H202 is toxic. Cells that produce H2O2 contain enzymes called catalases and peroxidases that mediate the reaction shown below: catalase 2H2O2 ⎯⎯→ 2H2O + O2 NOTE: Catalase is the name of an enzyme. Remember that enzymes are also catalysts (definition above) Question: How does the presence of these enzymes enable the cells to survive? (Answer on a separate sheet of paper) Materials: 8- 9 cm test tubes test tube holder 2 test tube racks 3-15 cm test tubes 1 razor blade splints matches 20 cm piece of dialysis membrane waxed paper for liver and potato 1 small funnel hot plate microscopes plastic pipette forceps marking pencil 1 250 ml beaker dropper bottle H2O small bottle of dilute HC1 small bottle of dilute NaOH thermometer 2- 500 beakers for water bath filter paper 1 small Erlenmeyer flask Opposite each sink: slides, coverslips, lens paper, potato juice, potato pieces, liver juice, liver pieces, stock H202, methylene blue, MnO2 (manganese dioxide), NaCl (salt), ice cubes. Procedure: Procedural Hints: Divide the work to be done according to your teacher's instructions. Each student's laboratory report should contain all problems investigated, and a section for Materials and Methods, Results, and Discussion. Problems to be investigated: (include answers to these questions in your lab write-up) Using the materials provided design experiments to answer the following questions. Prepare a hypothesis for Activity 16: Revised September 2003 Page 1 of 4 each experiment in the space provided, and write in the discussion section whether or not your hypothesis was valid. 1) How does the catalytic ability of inorganic and organic substances compare in the breakdown of hydrogen peroxide? a) In this investigation be sure to demonstrate that the material is only a catalyst, and not a reactant. b) Use one or two potato and liver chunks. c) How do extreme temperatures affect the ability of inorganic and organic catalysts to mediate their reactions? 2) How does high, neutral, and low pH affect the catalytic action of enzymes? 3) How does the size of the enzyme molecule compare to the size of its substrate molecule? a) In preparing your enzyme molecules, you must remove them from their cells. Should you use the potato/liver chunks or potato/liver juice? b) Be sure to demonstrate that these enzymes can work outside the living cell (in vitro). Problem: How do extreme temperatures affect the ability of inorganic and organic catalysts to mediate their reactions? Materials and Methods: A sample of the organic catalyst(s) and the inorganic catalyst(s) were obtained. Each sample was subjected to either an ice water bath or a boiling water bath. After this treatment, the samples were returned to room temperature and tested to see whether they retained their catalytic properties. This was accomplished by (describe experiments in your own words). Results: It was found that when inorganic catalysts are heated (describe your results). When they are chilled, (describe your results). When the organic catalysts from (potato or liver) are heated (describe your results). When chilled (describe your results). Use the Data Table below. Results may also be presented in graph form. Remember to label them Table or Figure 1 etc. and refer to the table or figure number in your results and discussion sections. Discussion: From these results, it is possible to make the following generalizations regarding the effect of temperature on inorganic and organic catalysts. Discuss the meaning of your results here. In some tests, the results were not as expected. This may be due to the following sources of error in the experimental procedure (list possible sources of error). Based on these findings, it is easy to see that high fever during illness may cause death because (put in your own words). Such temperature effects also explain the use of refrigeration to preserve food since (put in your own words). The following tables are for recording data. Give each table an appropriate title. Scoring system: 0 = no activity 1 = light activity 2 = moderate activity 3 = heavy activity Activity 16: Revised September 2003 Page 2 of 4 Table 1 Substance 1 Trial # 2 3 Conclusions Potato Liver Manganese dioxide (MnO2) NaCl Table 2 Substance Ice water Treatment Room temp. Boiling water Conclusions Potato Liver Manganese dioxide (MnO2) Table 3 Low pH (acid) Prior treatment Neutral pH (distilled H20) High pH (base) Substance Conclusions Potato Liver Table 4 Substance Reaction occurs Inside membrane Outside membrane Conclusions Potato juice Liver juice Activity 16: Revised September 2003 Page 3 of 4 Table 5: Class Data Group # Effect of pH on enzyme activity Low pH Potato Liver Potato/Liver/Manganese dioxide (MnO2) values Ice Water bath Room temp Boiling water High pH Neutral pH Bath L M L M P L M P Potato Liver Potato Liver P 1 2 3 4 5 6 7 8 9 Average Discussion Questions: Observations drawn from your investigations should enable you to answer the questions below, in addition to developing a coherent view of what enzymes are, what they do, and how they work. These are the ideas that should be emphasized in your Discussion. 1) What generalization can be made regarding the kinds of cells which have catalase, based on your laboratory, investigation? 2) Why do cells require comparatively small quantities of enzymes (as compared to substrate concentration) to carry out most metabolic functions? 3) Based on your observations of the effect of extreme temperatures on inorganic and organic catalysts, construct a graph that compares the effect of temperatures on the catalytic action of organic catalysts (enzymes) and inorganic catalysts. Use one set of axes, labeled with the appropriate independent and dependent variables. Use a solid line and a broken line to make the distinction between the two lines on the graph. Alternately, you can use a different color for each line on the graph. Application of Concept: 1) Why is an excessively high body temperature often life threatening? 2) Why are refrigeration and pickling successful methods of food preservation? HINT: Food spoilage is the result of the metabolic activity of bacteria. WRITE UP YOUR LABORATORY REPORT USING THE RESEARCH PAPER FORMAT SAMPLE LABORATORY REPORT ON ONE INVESTIGATION Activity 16: Revised September 2003 Page 4 of 4