Hydrolysis of Ions: pH Calculations & Common Ion Effect
advertisement
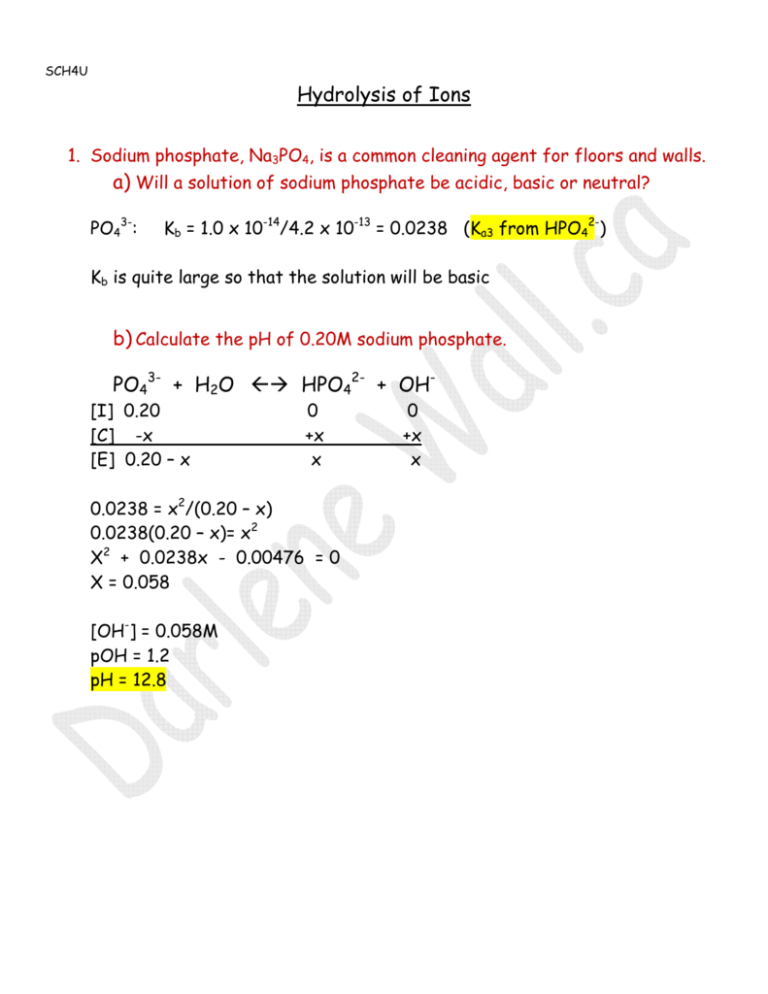
SCH4U Hydrolysis of Ions 1. Sodium phosphate, Na3PO4, is a common cleaning agent for floors and walls. a) Will a solution of sodium phosphate be acidic, basic or neutral? PO43-: Kb = 1.0 x 10-14/4.2 x 10-13 = 0.0238 (Ka3 from HPO42-) Kb is quite large so that the solution will be basic b) Calculate the pH of 0.20M sodium phosphate. PO43- + H2O HPO42- + OH[I] 0.20 [C] -x [E] 0.20 – x 0 +x x 0.0238 = x2/(0.20 – x) 0.0238(0.20 – x)= x2 X2 + 0.0238x - 0.00476 = 0 X = 0.058 [OH-] = 0.058M pOH = 1.2 pH = 12.8 0 +x x 2. Calculate the pH of a solution made by dissolving 8.20 g of NaCH3CO2 in water to a final volume of 500.0 mL. nNaCH3O2- = 8.20 g/82.034 = 0.10 mol [CH3CO2-] = 0.10 mol/0.500L = 0.20M CH3CO2- + H2O [I] 0.20 [C] -x [E] 0.20 – x CH3CO2H 0 +x x + OH0 +x x Kb = 1.0 x 10-14/ 1.8 x 10-5 = 5.6 x 10-10 5.6 x 10-10 = x2/0.20 X2 = 1.12 x 10-10 X = 1.1 x 10-5 [OH] = 1.1 x 10-5 M pOH = 5.0 pH = 9.0 *insignificant 3. How many grams of potassium nitrite, KNO2, must be dissolved in 1.0 L of water to make a solution with a pH of 8.8? Kb = 1.0 x 10-14/7.2 x 10-4 = 1.39 x 10-11 pH = 8.8 therefore pOH = 5.2 [OH-] = 6.3 x 10-6 M NO2- + H2O [I] 0.20 [C] -x [E] 0.20 – 6.3 x 10-6 HNO2 + 0 +6.3 x 10-6 6.3 x 10-6 *insignificant 1.4 x 10-11 = (6.3 x 10-6)2/x x = 2.8 Therefore [KNO2] = 2.8 mol/L mKNO3 = 2.8 mol/L x 1.0L x 85.11 g/mol = 238 g OH0 +6.3 x 10-6 6.3 x 10-6 4. Calculate the pH of a 0.15M solution of a) NH4NO3 a) NH4+ / NO3Ka NH4+ = 10-14/(1.8 x 10-5) = 5.6 x 10-10 Kb NO3- = 10-14/ very large value = a value less than 10-14 therefore negligible Since Ka for NH4+ > Kb for NO3- , NH4NO3 will be acidic NH4+ [I] 0.15 [C] -x [E] 0.15 – x NH3 0 +x x 5.6 x 10-10 = x2/0.15 X = 9.2 x 10-6 = [H+] pH = 5.0 + H+ 0 +x x b) NaHC2O4 Na+/ HC2O4Na+ can’t act as an acid HC2O4- is amphoteric – it can act as an acid or base – which will it do to the greatest extent? As an acid: HC2O4- H+ + C2O42- Ka = Ka2 = 5.4 x 10-5 As a base: HC2O4- + H2O H2C2O4 + OHKb = 10-14/Ka1 = 10-14/5.4 x 10-2 = 1.9 x 10-13 Ka >> Kb therefore it will act as an acid HC2O4- H+ + C2O42[I] 0.15 0 0 [C] -x +x +x [E] 0.15 – x x x -5 2 5.4 x 10 = x / 0.15 X = 2.8 x 10-3 = [H+] pH = 2.5 Ka 5.4 x 10-5 5. 25.0 mL of 0.10M acetic acid is titrated with 25.0 mL of 0.10M NaOH. a) Write the chemical equation for the titration reaction. CH3CO2H + NaOH NaCH3CO2 + H2O b) Calculate the concentration of the resulting solution of the salt, sodium acetate. n NaCH3CO2 = nNaOH = 0.10 mol/L x 0.025L = 0.0025 mol Therefore nCH3CO2- = 0.0025 mol (1:1) CNaCH3CO2 = 0.0025 mol/0.050L = 0.050 M c) Calculate the pH of the resulting solution. CH3CO2- + H2O CH3CO2H + OH[I] 0.050 [C] -x [E] 0.20 – x 5.6 x 10-10 0 +x x = x2/0.050 X = 5.3 x 10-6 0 +x x insignificant [OH-] = 5.3 x 10-6 M pOH = 5.3 pH = 8.7 Common Ion 6. A solution is made by dissolving 6.8 g of NH3 and 10.7 g of NH4Cl in water with a final volume of 250 mL. The Kb for NH3 is 1.8 x 10-5. Calculate the OHconcentration of the solution. The common ion will be NH4+ (from ionization of NH3 and from the salt NH4Cl) NH3 + H2O NH4+ + OH- Kb = 1.8 x 10-5 nNH3 = 6.8 g/17.034 = 0.40 mol [NH3] = 0.40mol/0.250L = 1.6M nNH4Cl = 10.7g/53.49 = 0.20mol [NH4+] = 0.20 mol/0.250L = 0.80M [I] [C] [E] NH3 + H2O 1.6 -x 1.6 – x 1.8 x 10-5 = 0.80x/1.6 X = 3.6 x 10-5 [OH-] =3.6 x 10-5 M NH4+ + OH0.80 0 +x +x 0.80 + x x Kb = 1.8 x 10-5 insignificant 7. a) Calculate the pH of a 0.60M solution of acetic acid. CH3CO2H CH3CO2- + H+ [I] [C] [E] 0.60 -x 0.60 – x 0 +x x 0 +x x insignificant 1.8 x 10-5 = x2/0.60 X = 0.0033 = [H+] pH = 2.5 b) Determine the mass of sodium acetate that must be dissolved in 1.0 L of 0.60M acetic acid to bring the pH to 6.0. pH = 6.0 [H+] = 10-6 CH3CO2H CH3CO2- + H+ [I] [C] 0.60 - 10-6 x +10-6 0 +10-6 [E] 0.60 - 10-6 x + 10-6 10-6 insignificant 1.8 x 10-5 = 10-5x /0.60 X = 10.8 = [CH3CO2-] MCH3CO2- = 10.8 mol/L x 1.0L x 82.03 g/mol = 886g 8. How many grams of sodium formate, NaCHO2, would have to be dissolved in 1.0 L of 0.12M formic acid to make a solution with a pH of 3.80. Ka for formic acid is 1.8 x 10-4. [I] [C] [E] HCHO2 0.12 - 1.6 x10-4 0.12 – 1.6 x 10-4 CH2O- + H+ x 0 -4 +1.6 x 10 +1.6 x 10-4 x +1.6 x 10-4 1.6 x 10-4 1.8 x 10-4 = x(1.6 x 10-4)/0.12 x = 0.14 mol/L = [CH2O-] m = 0.14mol/L x 1.0 L x 68.01 g/mol = 9.5 g