Warfarin Interactions With Substances Listed in Drug Information

nature publishing group articles
Warfarin Interactions With Substances Listed in Drug Information Compendia and in the
FDA-Approved Label for Warfarin Sodium
M Anthony
1
, K Romero
1
, DC Malone
2
, LE Hines
3
, L Higgins
4
and RL Woosley
1 interactions of warfarin with other drugs or substances can pose a serious problem. We assessed three drug information compendia—Clinical pharmacology, epocrates, and micromedex—and the warfarin sodium (Coumadin) product label
(august 2007) approved by the us Food and Drug administration for listings of interactions between warfarin and drugs, biologics, foods, and dietary supplements. The drug information compendia and warfarin label differed greatly as to the total number of substances that interact with warfarin. of a total of 648 entries from the four sources, only 50 were common to all the sources. The types of substances listed as interacting with warfarin were entire classes of drugs, individual drugs, and combinations; biologics; dietary supplements; foods; alcohol; and tobacco. These sources were then examined for classification by severity of interaction and the underlying evidence base. This study provides evidence that there is little concordance among commonly used drug compendia and product labels with respect to interactions involving warfarin.
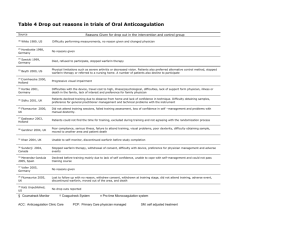
Although warfarin can be a lifesaving medication, its clinical management is challenging because of its narrow therapeutic index and wide dosing range (up to 20-fold variability), 1 which make it necessary to frequently monitor the state of anticoagulation as measured by the international normalized ratio (INR) in order to make dose adjustments.
2 There are many factors that contribute to the variability in dose requirements for and response to warfarin. Age, sex, and weight (or an indication of body mass) account for 10–20% of the interpatient variability in dose requirements.
3 In addition, there is increasing evidence that genetic polymorphisms—especially in cytochrome P450 isoenzyme 2C9 and vitamin K epoxide reductase complex
sub unit 1—affect dosing and may account for up to 15 and 25%, respectively, of the variability in dose requirements.
4,5 However, an additional major influence on variability is the interaction of warfarin with other medications, dietary supplements, foods, and alcohol.
6,7 Studies have reported that a high percentage
(68–84%) of patients on warfarin receive potentially interacting medications.
8–10 Even after a stable INR and therapeutic dose of warfarin have been reached, changes in medications, diet, and other ingested substances can result in either a decrease in
INR, which is associated with reduced drug efficacy and can lead to increased clotting, or an increase in INR, which can result in increases in adverse events such as bleeding.
11 For example, the rate of serious gastrointestinal bleeding among persons exposed to a warfarin–nonsteroidal anti-inflammatory drug inter action is fivefold higher than in persons on warfarin alone.
Of 25 potential drug interactions thought to be clinically significant, 6 involved warfarin. An additional list identifying clinically important interactions included that between warfarin and nonsteroidal anti-inflammatory drugs.
Determining which medications, dietary supplements, and foods interact with warfarin is a complicated task. With regard to drug interaction compendia, other studies have highlighted substantial differences with respect to classification of interaction severity.
13
12
Because interactions with warfarin present a serious risk to patients, agreement among the various drug information sources is essential. The purpose of this study was to examine commonly used drug compendia and the warfarin (Coumadin brand) product label to compare the listings of substances that have potential to interact with warfarin.
Results
Number of entries
Three drug information compendia (Clinical Pharmacology, ePocrates, and Micromedex) and the Coumadin label differed as to the number of medications listed as having potential to interact with warfarin. As of March 2008, there were 182 entries
1 The Critical Path Institute, Tucson, Arizona, USA; 2 Department of Pharmacy Practice and Science, College of Pharmacy, University of Arizona, Tucson, Arizona, USA;
3 Center for Health Outcomes and PharmacoEconomic Research, College of Pharmacy, University of Arizona, Tucson, Arizona, USA;
College of Medicine, University of Arizona, Tucson, Arizona, USA. Correspondence: M Anthony ( manthony@c-path.org
)
4 Valley Fever Center for Excellence,
Received 12 December 2008; accepted 20 April 2009; advance online publication 8 July 2009. doi: 10.1038/clpt.2009.95
CliniCal pharmaCology & TherapeuTiCs 1
articles of potential warfarin–drug interactions listed in ePocrates, 201 entries in Clinical Pharmacology, 295 in the warfarin label, and 427 in Micromedex. With regard to unique chemical entities, Clinical Pharmacology listed 84 drugs as interacting with warfarin, ePocrates listed 90, the label contained 148, and
Micromedex had 245.
A total of 91 dietary supplements were listed as interacting with warfarin, as well as a total of 16 items of food, ethanol/ alcohol, and tobacco. Micromedex listed 53 dietary supplements and 11 foods, and the Coumadin label was similar, listing 50
dietary supplements and 10 foods. Clinical Pharmacology listed 25 dietary supplements and 5 foods, and ePocrates listed
6 dietary supplements and 1 food.
With regard to dietary supplements, foods, and recreational substances, only vitamin K and ethanol were listed in all four sources as interacting with warfarin. The result for the global
Fleiss’ κ concordance for dietary supplements was poor
(−0.139). The pairwise concordance with Cohen κ coefficient for
dietary supplements in Micromedex vs. Clinical Pharmacology was −0.064; vs. ePocrates it was −0.096; and vs. the warfarin label it was −0.229. The Cohen κ for dietary supplements in
Clinical Pharmacology vs. ePocrates was 0.098 and vs. the label it was −0.073. The corresponding coefficient for dietary supplements in ePocrates vs. the label was −0.052. The global Fleiss
κ for food was also poor, at −0.167. The highest result for food or any other set of items was that for Micromedex vs. Clinical
Pharmacology—0.342—denoting fair agreement. By contrast, the result for food in Micromedex vs. ePocrates was 0.059, and vs. the label it was −0.517. The Cohen κ for food in Clinical
Pharmacology vs. ePocrates was −0.116, and vs. the label it was −0.257. The Cohen κ coefficient for food in ePocrates vs. the label was 0.077.
Degree of concordance
A total of 648 entries, from all four sources, were listed as having
potential to interact with warfarin. Table 1 lists products that
were common to all four sources. There were 50 entries in this category, of which 47 were medications and one a biologic (influenza vaccine); the remaining 2 were vitamin K and ethanol.
Table 2 shows the percentage of substances common to all four
sources—only 7.7% of the substances were listed in all four. More than half (57.3%) of the substances were present in only one source; 22% were listed in two sources and 13% in three.
There was very little agreement among the compendia and the label with respect to the listing of drugs according to Landis and
Koch’s standards for strength of agreement for κ coefficients: ≤0 = poor, 0.01–0.20 = slight, 0.21–0.40 = fair, 0.41–0.60 = moderate, 0.61–0.80 = substantial, and 0.81–1 = almost perfect.
14 The result for the Fleiss’ κ concordance coefficient among all databases was −0.026. As for the pairwise concordance measured with Cohen’s
κ coefficient, Clinical Pharmacology–ePocrates had the highest coefficient (0.242), with the second highest result (0.107) being obtained for the Clinical Pharmacology–label pair. The remaining coefficients between the sources were, in descending order of concordance, 0.015, −0.039, −0.052, and −0.172 for the ePocrates– label, Clinical Pharmacology–Micromedex, ePocrates–Micromedex, and Micromedex–label pairs, respectively.
table 1 substances listed in all four sources
Acetaminophen Fenofibrate Micronazole Rifampin
Allopurinol
Amiodarone
Fluoxetine
Flutamide
Azathioprine Fluvastatin
Carbamazepine Fluvoxamine
Cefazolin Gemfibrozil
Chloramphenicol Glucagon
Cimetidine
Dicloxacillin
Griseofulvin
Heparin
Nafcillin
NSAIDs
Paroxetine
Phenytoin
Disulfiram
Ethanol
Influenza vaccine Quinine
Lovastatin Raloxifene
Ethacrynic acid Methylphenidate Ranitidine
Ezetimibe Metronidazole
NSAIDs, nonsteroidal anti-inflammatory drugs.
Salicylates
Sertraline
Simvastatin
Sucralfate
Propafenone Sulfinpyrazone
Propoxyphene Tamoxifen
Propranolol
Quinidine
Thrombolytics
Tramadol
Vitamin K
Zafirlukast
Zileuton severity classification
The types of substances listed as having interactions with
warfarin were drugs, biologics, dietary supplements, foods, alcohol, and tobacco, with differences with respect to the
spec ificity of the terms. For example, there were broad categories such as “food,” “high-protein foods,” “foods high in vitamin
K” and “enteral feedings/nutrition,” as well as individual foods.
Drugs were listed as individual drugs, drug combinations, and drug classes. Clinical Pharmacology used drug class frequently whereas Micromedex was drug specific. The label used both drug class and individual drugs. Biologics were listed as individual biological products such as monoclonal antibodies,
vaccines, and recombinants. Dietary supplements were listed as individual products.
Each drug information source used a different terminology to describe interactions with warfarin. ePocrates used five categories:
“Contraindicated,” “Avoid/Use Alternative,” “Monitor/Modify
Treatment,” “Therapeutic Advantage,” and “Caution Advised.”
Clinical Pharmacology used four terms: “Very High Severity,”
“High Severity,” “Moderate Severity,” and “Low Severity.”
Interactions labeled “Very High Severity” comprised those that must almost always be avoided. Interactions labeled “High
Severity” were those that commonly result in either an increase or a decrease in the effect of at least one drug—in most cases, this change could be expected to be harmful. “Moderate Severity” interactions were characterized as producing a change in the effect of one or both drugs, but the clinical consequences were often not serious. A rating of “Low Severity” indicated that a slight table 2 entries common to different sources
Common in
All four sources
Only three sources
Only two sources
Only one source number
50
85
143
370 percentage
7.70
13.00
22.00
57.30
2 www.nature.com/cpt
articles risk for an interaction exists, and that such an interaction may affect one or both drugs but rarely causes significant problems.
Clinical Pharmacology also gave a brief summary of the supporting literature with references. Micromedex classified interactions into three severity categories: “Major,” “Moderate,” and “Minor.”
Each of the Micromedex listings had additional information as to grade of evidence to support the risk of an interaction, using a
rating scale of “excellent, good, or fair,” and a brief general summary of the clinical outcome that was most commonly described as an increased risk of bleeding or decreased anticoagulation.
In contrast, the label did not use a severity classification but instead had two sections based on the ability of the substance to increase or decrease the prothrombin time/INR response. Drug or biologic classes were listed first, followed by individual drugs or biologics. Fifty-seven drug classes and 133 individual drugs were listed as having the effect of increasing the prothrombin time/INR, and 24 drug classes and 44 individual drugs were listed as capable of decreasing the prothrombin time/INR. For some drugs, such as cyclophosphamide, moricizine, and propylthiouracil, both an increase and a decrease in prothrombin time/INR responses were reported as possible. Botanicals were listed in a separate section. Seventy-four botanicals were categorized as having potential anticoagulant effect and anticoagulant, antiplatelet, fibrinolytic, or coagulant properties.
Table 3 Acetaminophen and combination products listed potential agents interacting with warfarin
Acetaminophen
Acetaminophen/ butalbital
Acetaminophen/ caffeine/CNS depressant combinations
Acetaminophen/ codeine phosphate
Clinical pharmacology epocrates micromedex label
+
0
+
+
+
0
+
0
0
0
+
0
0
+
0
0
0 0 + 0 Acetaminophen/ dichloralphenazone/ isometheptene mucate
Acetaminophen/ hydrocodone bitartrate
Acetaminophen/ opiate combos
0
0
0
+
+
0
0
0
0 0 + 0 Acetaminophen/ oxycodone hydrochloride
Acetaminophen/ propoxyphene
Acetaminophen/ tramadol
0
0
+
+
+
+
0
0
CNS, central nervous system.
Drug classes, individual drugs, and drug combinations
In these four sources, a drug could be listed in terms of a drug class, as an individual drug, or both. The Coumadin label included β-adrenergic blockers as a drug class but also three individual β-blockers (atenolol, propranolol, and stanozolol). Of these, all four sources listed propranolol as a drug that interacts with warfarin. Clinical Pharmacology included the drug class
quinolones, without naming any individual drugs. Both ePocrates and Micromedex listed nine individual quinolones, but the lists of drugs were not identical. The label mentioned both the drug class (quinolones) and five individual drugs— ciprofloxacin, levofloxacin, nalidixic acid, norfloxacin, and ofloxacin. For hydroxymethylglutaryl coenzyme A reductase inhibitors
( statins), Clinical Pharmacology included the drug class and three
individual drugs—fluvastatin, lovastatin and simvastatin—that were also listed in ePocrates and Micromedex but without mention of the drug class. The Coumadin label mentioned the drug class as well as atorvastatin, cerivastatin, fluvastatin, lovastatin, pravastatin, and simvastatin, but not rosuvastatin.
There were also differences with regard to drugs and
combination products. Each of the four sources listed
acet aminophen as interacting with warfarin ( Table 3 ). However,
acetaminophen-combination products were included in
ePocrates and Micromedex; acetaminophen-plus-hydrocodone was listed only in Micromedex.
Different terms were used to refer to the same compounds.
“Antiretroviral protease inhibitors” was in one source, whereas another source used “protease inhibitors.” Micromedex and the label used the term “thyroid”; however, the label also referred to
“thyroid drugs,” whereas Clinical Pharmacology and ePocrates referred to “thyroid hormones.”
Discussion
In 2005, an average of 12 prescriptions were dispensed for every adult and child in the United States, and an average of 17 prescriptions were dispensed per person >65 years of age.
15 This level of medication consumption creates considerable potential for adverse events resulting from pharmacokinetic and pharmacodynamic interactions between medications. In fact for 6 of 12 medications removed from the market, the harmful effects were often the result of drug interactions.
16 According to studies on adverse drug reactions, one of the largest subsets of preventable harm is that caused by drug–drug interactions.
17–19
Prescribers need better information to reduce drug interactions, which needs to be articulated as a major focus. The results reported in this study call attention to the problems of determining clinically significant drug interactions, namely, the large
numbers of substances listed as interacting with warfarin and the lack of concordance among the sources and the product label.
There are many reasons for this lack of agreement, including variability in methodologies; the use of diverse and, at times, vague terms for products; listing individual drugs, combination products, or drug classes in an arbitrary manner; differences in severity
classifications; the lack of data on the potential for and frequency of occurrence of interactions; the application of interaction warnings to all drugs in a class, even when not appropriate; and the lack or sparseness of documentation on the underlying evidence base, even for substances with the highest severity warnings. All these contribute to explaining the low percentage of entries common to all information sources (7%) and the high percentage of entries listed in only one of the four sources (57.30%).
CliniCal pharmaCology & TherapeuTiCs 3
articles
Some drug information sources assign interactions to all
products within a therapeutic class, such as β-blockers, nonsteroidal anti-inflammatory drugs, and quinolones, whereas others do not. This approach leads to complications if there is not sufficient evidence to assign an interaction to an entire class.
Of several cases, the examples of anticoagulants and hydroxymethylglutaryl coenzyme A inhibitors (statins) are illustrative.
Pharmacodynamic interactions of two anticoagulants used together could be considered a sound reason to list the drug class instead of individual agents. On the other hand, the available evidence suggests that not all statins have the same potential for interaction with warfarin; e.g., pravastatin does not interact with warfarin to a significant extent.
20–22 In the case of statins, listing the drug class but not the individual agents for which there is a reasonable degree of evidence of interaction could prove to be misleading. Listing statins as a class would be sensitive but not specific, and listing individual drugs would be better. All this contributes to the low overall and pairwise concordance results.
Practitioners need to know whether a source is drug- specific or whether it lists drug classes. Such details are not explicitly
provided in any source. Also, practitioners need to know whether a potential warfarin interaction is a drug class effect and then search for either the drug class or the individual product.
The reference sources used terms that lack specificity. For example, thyroid was variously termed “thyroid,” “thyroid drugs,” and “thyroid hormones.” “Thyroid” mentioned by itself can mean the gland. The product called “desiccated thyroid” is a combination of levothyroxine (T4) and liothyronine (T3) derived from porcine thyroid glands. “Thyroid hormones” could mean any of the possible thyroid hormone combination and forms available for hypothyroidism, such as desiccated thyroid, levothyroxine, and liotrix. The term “thyroid drugs” may include drugs used to treat hypothyroidism (several forms of thyroid hormones) or hyperthyroidism (antithyroid agents such as methimazole). The various options for hormone replacement for hypothyroidism include thyroid hormone T4 alone, T3 alone, or a combination, in different forms and with different origins.
In this case, the interaction with warfarin is proposed as being caused by an increase in catabolism of vitamin K–dependent clotting factors, which can increase the anticoagulant effect.
With thyroid suppression for hyperthyroidism, warfarin can interact variably with methimazole and propylthiouracil. These medications tend to induce a decrease in the anticoagulant effect because the antithyroid medications reverse the hyperthyroid state. However, rare cases of excessive anticoagulation and bleeding have occurred with propylthiouracil.
23–25 Therefore, specifying the therapeutic purpose of the product would be helpful. Prescribers need to use several terms in searching any one source, in order to ensure that a potential interaction is not overlooked. Reconciliation of the inconsistencies discussed and the development of a standardized risk-stratification process are necessary to achieving a higher rate of agreement among the different databases and product labels.
There are almost one thousand scientific reports on drug interactions with warfarin. Evidence for determining interactions comes from many sources—animal and in vitro studies, case reports, studies in healthy volunteers, and extrapolations from biologically plausible assumptions based on pharmacokinetic or pharmacodynamic studies. More rarely, epidemiologic or randomized controlled clinical trials, as well as postmarketing reports submitted to the US Food and Drug Administration
Adverse Events Reporting System and the British Committee on
Safety of Medicines, also contain reports of interactions between warfarin and other drugs or substances. However, the underlying evidence for a product’s potential to produce interactions with warfarin was not explicit in the drug information sources.
These findings represent a major problem for prescribers in treating patients on warfarin who have comorbid conditions: how best to use the large volume of information, reconcile inconsistencies and vagaries, and determine which potential interactions are clinically significant. Certainly, inconsistencies in lists of drug interactions translate into suboptimal practice tools for prescribers, pharmacists, and patients. In nursing homes,
25% of warfarin prescribing errors (that were either preventable adverse warfarin-related events or potential adverse warfarinrelated events) were caused by prescribing a drug known to
27–30 interact with warfarin.
26 Alert fatigue is well documented.
Prescription data from a major pharmacy benefit manager reveal that 374,000 of 46 million participants were exposed to a potential drug interaction of clinical importance, and the largest number of these incidents involved coprescription of warfarin with nonsteroidal anti-inflammatory drugs.
31
Because this is a complex problem, several steps and approaches are necessary. A first step could be to improve the consistency in use and specificity of terms for drugs; this will be difficult to achieve without guidance from an authorized body.
Another major step would be to document the evidence base by addressing the adequacy of the literature. This would start with a review of the scientific literature, with ratings for the type of study and the quality and concordance of the evidence, followed by an expert panel review and development of a consensus.
12
The substances listed in Table 1 could constitute a priority for
a literature search. Information on the pharmacokinetics and pharmacodynamics of the drugs is useful. Other information important for clinicians are the potential for drug interactions and the frequency of occurrence of such interactions. Specificity on the strength of the interaction and its potential to cause harm are needed to assist in appropriate management. Another consideration is the classification of the severity of the adverse event. Drug information sources should be more explicit in their approach to assigning warning labels to specific interactions. At a minimum, the product labels should be regularly updated as new drug information becomes available.
Patient care requires not only information about potential interactions but recommendations for how to manage them
clinically—should the drug be avoided, should it be used only under certain circumstances, or should available alternatives be considered? As more clinicians are using electronic prescribing systems, it is imperative that drug interaction alerts provide useful, clinically relevant information. Issuing trivial alerts or warnings about inappropriate class effects is likely to result in these clinical decision support systems being disabled because they “cry wolf”
4 www.nature.com/cpt
articles too often. Practitioners need better information and tools to help with decision making regarding the risks of interactions with
warfarin, so as to avoid harmful prescribing and identify adequate therapeutic alternatives. Although we are specifically addressing warfarin in this study, we believe that the same issues apply to the entire field of drug interactions. When a better evidence base and improved decision tools become available, the next challenges will be changing the prescribing behavior of practitioners and implementing systems approaches.
MethoDs
We conducted a comparison of three major, commonly used drug
information compendia—Clinical Pharmacology, ePocrates, and
Micromedex—for their listings of potential interactions between warfarin and drugs, biologics, foods, and dietary supplements. Research shows that prioritizing warning information in labeling has an impact on reducing preventable adverse events (http://www.fda.gov/fdac/ features/2006/206_format.html). We therefore evaluated the most recent
Coumadin product label approved by the Food and Drug Administration
(16 August 2007) 32 with respect to how interactions of other products with warfarin were reported. We compiled the exact listings from these four sources into a comprehensive table in Microsoft Excel (Microsoft,
Redmond, WA, 2003) and SPSS (SPSS, Chicago, IL, 2007). For the purpose of comparing the compendia and the product label, and in view of the fact that each source has its own categorization of different levels of potential interactions, each drug or product was transformed into a binary variable (present and absent). This binary variable was used to determine the level of concordance between databases. Fleiss’ κ coefficient was utilized to assess the overall concordance, and Cohen’s κ coefficient was used to determine pairwise concordance between individual databases.
After entering data from the three drug compendia and the label into the Excel and SPSS databases, the results were compared in order to determine similarities, differences, and inconsistencies regarding the substances listed and how they were listed. We also reviewed information from these sources on the ranking system for the severity of the inter action and documentation of details from the underlying evidence base.
AckNowleDgMeNts
This project was supported by grant U18HS017001 from the Agency for
Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official view of the
Agency for Healthcare Research and Quality.
coNflict of iNteRest
The authors declared no conflict of interest.
© 2009 American Society for Clinical Pharmacology and Therapeutics
1. Anderson, J.L. et al . Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation 116 ,
2563–2570 (2007).
2. Ansell, J.E. et al . Consensus guidelines for coordinated outpatient oral anticoagulation therapy management. Anticoagulation Guidelines Task
Force. Ann. Pharmacother.
31 , 604–615 (1997).
3. Sconce, E.A. et al . The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood 106 , 2329–2333 (2005).
4. Aquilante, C.L. et al . Influence of coagulation factor, vitamin K epoxide reductase complex subunit 1, and cytochrome P450 2C9 gene polymorphisms on warfarin dose requirements. Clin. Pharmacol. Ther 79 ,
291–302 (2006).
5. Wadelius, M. et al . Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics J.
5 , 262–270 (2005).
6. Greenblatt, D.J., von Moltke, L.L. Interaction of warfarin with drugs, natural substances, and foods. J. Clin. Pharmacol.
45 , 127–132 (2005).
7. Holbrook, A.M. et al . Systematic overview of warfarin and its drug and food interactions. Arch. Intern. Med.
165 , 1095–1106 (2005).
8. Kotirum, S. et al . Utilization review of concomitant use of potentially interacting drugs in Thai patients using warfarin therapy. Pharmacoepidemiol.
Drug Saf.
16 , 216–222 (2007).
9. Snaith, A. et al . The potential for interaction between warfarin and coprescribed medication: a retrospective study in primary care. Am. J.
Cardiovasc. Drugs 8 , 207–212 (2008).
10. Verhovsek, M. et al . Quality of anticoagulation and use of warfarin-interacting medications in long-term care: a chart review. BMC Geriatr.
8 , 13 (2008).
11. Schulman, S., Beyth, R.J., Kearon, C., Levine, M.N. American College of Chest
Physicians. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical
Practice Guidelines (8th Edition). Chest 133 (6 suppl.), S257–S298 (2008).
12. Malone, D.C. et al . Identification of serious drug-drug interactions: results of the partnership to prevent drug-drug interactions. J. Am. Pharm. Assoc. (2003)
44 , 142–151 (2004).
13. Abarca, J. et al . Concordance of severity ratings provided in four drug interaction compendia. J. Am. Pharm. Assoc. (2003) 44 , 136–141 (2004).
14. Landis, J.R. & Koch, G.G. The measurement of observer agreement for categorical data. Biometrics.
33 , 159–174 (1977).
15. 2005 Community Pharmacy Prescription Results. Association of Chain Drug
Stores (2006).
16. Chen, M.C., Huang, S.M., Beitz, J. & Temple, R. Did drugs withdrawn in recent years have greater risk in women? Findings from postmarketing reporting of adverse events data. Clear Science Communication Award – 2003 FDA
Science Forum. <http://www.accessdata.fda.gov/ScienceForums/forum03/
W-08.htm> (2008).
17. Leape, L.L. et al . Systems analysis of adverse drug events. ADE Prevention
Study Group. JAMA 274 , 35–43 (1995).
18. Bates, D.W. et al . The impact of computerized physician order entry on medication error prevention. J. Am. Med. Inform. Assoc.
6 , 313–321 (1999).
19. Bates, D.W. et al . The costs of adverse drug events in hospitalized patients.
Adverse Drug Events Prevention Study Group. JAMA 277 , 307–311 (1997).
20. Hickmott, H., Wynne, H. & Kamali, F. The effect of simvastatin co-medication on warfarin anticoagulation response and dose requirements. Thromb.
Haemost.
89 , 949–950 (2003).
21. Kim, M.J. et al . Effects of fluvastatin and cigarette smoking on CYP2C9 activity measured using the probe S-warfarin. Eur. J. Clin. Pharmacol.
62 , 431–436
(2006).
22. Ahmad, S. Lovastatin. Warfarin interaction. Arch. Intern. Med.
150 , 2407
(1990).
23. Greenstein, R.H. Hypoprothrombinemia due to propylthiouracil therapy.
JAMA 173 , 1014–1015 (1960).
24. Gotta, A.W., Sullivan, C.A., Seaman, J. & Jean-Gilles, B. Prolonged intraoperative bleeding caused by propylthiouracil-induced hypoprothrombinemia.
Anesthesiology 37 , 562–563 (1972).
25. Gilbert, D.K. Hypoprothrombinemia as a Complication of Propylthiouracil.
JAMA 189 , 855 (1964).
26. Gurwitz, J.H. et al . The safety of warfarin therapy in the nursing home setting.
Am. J. Med.
120 , 539–544 (2007).
27. Weingart, S.N. et al . Physicians’ decisions to override computerized drug alerts in primary care. Arch. Intern. Med.
163 , 2625–2631 (2003).
28. Soumerai, S.B. & Lipton, H.L. Computer-based drug-utilization review—risk, benefit, or boondoggle? N. Engl. J. Med.
332 , 1641–1645 (1995).
29. Payne, T.H. et al . Characteristics and override rates of order checks in a practitioner order entry system. Proc. AMIA Symp.
2002 , 602–606.
30. Committee on Quality of Health Care in America. Institute of Medicine.
Crossing the Quality Chasm: A New Health System for the 21st Century
(National Academy Press, Washington, D.C., 2001).
31. Malone, D.C. et al . Assessment of potential drug-drug interactions with a prescription claims database. Am. J. Health Syst. Pharm.
62 , 1983–1991
(2005).
32. Coumadin [prescribing information]. Princeton, NJ: Bristol-Myers Squibb
Company, 2007.
CliniCal pharmaCology & TherapeuTiCs 5